Our Products
Our Products
At ABC International, we are dedicated to developing, manufacturing, sourcing and delivering only the highest quality pharmaceutical products along with renowned partners across the globe. Our unwavering mission is to bridge the gaps in the markets we serve in, with a primary focus on Myanmar.
We understand that in healthcare, excellence is non-negotiable. That’s why we have established partnerships with industry leaders who share our commitment to uncompromising quality and patient well-being. Our meticulously curated range of pharmaceutical and OTC products encompasses a wide spectrum of categories, ensuring that we cater to the diverse medical needs of our patients.
Whether it’s ground-breaking OTC products, cutting-edge medical devices, or critical healthcare essentials, we scour the world to bring the very best. Our tireless dedication to product quality ensures access to the Novel & effective quality pharmaceuticals.
At ABC International, we not only provide medications, we deliver a promise of quality, efficacy and safety.
Our Products
At ABC International, we are dedicated to developing, manufacturing, sourcing and delivering only the highest quality pharmaceutical products along with renowned partners across the globe. Our unwavering mission is to bridge the gaps in the markets we serve in, with a primary focus on Myanmar.
We understand that in healthcare, excellence is non-negotiable. That’s why we have established partnerships with industry leaders who share our commitment to uncompromising quality and patient well-being. Our meticulously curated range of pharmaceutical and OTC products encompasses a wide spectrum of categories, ensuring that we cater to the diverse medical needs of our patients.
Whether it’s ground-breaking OTC products, cutting-edge medical devices, or critical healthcare essentials, we scour the world to bring the very best. Our tireless dedication to product quality ensures access to the Novel & effective quality pharmaceuticals.
At ABC International, we not only provide medications, we deliver a promise of quality, efficacy and safety.

Quality Healthcare Products For Every Need
ABC International works with global majors to bridge the therapy gaps in Myanmar by providing quality pharmaceuticals.

Novapressin
Novapressin
Novapressin contains
Terlipressin Acetate (triglycyl¬lysine-vasopressin) 1mg
Uses
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Novapressin
Novapressin
Novapressin contains
Terlipressin Acetate (triglycyl¬lysine-vasopressin) 1mg
Uses
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia


Xavor 50 mg Tab
Xavor
Each film coated tablet contains:
Losartan potassium 50mg & Hydrochlorothiazide 12.5mg; Inactive Ingredients: Colloidal Anhydrous Silica; Magnesium Stearate; Sodium Starch Glycolate; Talcum; Microcrystalline Cellulose; Opadry Orange 85G53561; Carnauba Wax.
Uses
XAVOR-DIU is indicated for the treatment of hypertension.
XAVOR-DIU is indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy.
Side Effect
Edema, Palpitation, Dizziness, Abdominal pain, Back pain, cough, sinusitis
Bilirubin increased (serum), BUN increased, hematocrit decreased, hemoglobin decreased, hyper-/hypotension hyponatremia, liver enzymes increased, rhabdomyolysis, serum creatinine increased, thrombocytopenia.

Xavor DIU Tab
Xavor
Each film coated tablet contains:
Losartan potassium 50mg & Hydrochlorothiazide 12.5mg; Inactive Ingredients: Colloidal Anhydrous Silica; Magnesium Stearate; Sodium Starch Glycolate; Talcum; Microcrystalline Cellulose; Opadry Orange 85G53561; Carnauba Wax.
Uses
XAVOR-DIU is indicated for the treatment of hypertension.
XAVOR-DIU is indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy.
Side Effect
Edema, Palpitation, Dizziness, Abdominal pain, Back pain, cough, sinusitis
Bilirubin increased (serum), BUN increased, hematocrit decreased, hemoglobin decreased, hyper-/hypotension hyponatremia, liver enzymes increased, rhabdomyolysis, serum creatinine increased, thrombocytopenia.

Aurora 5 mg Tab
Aurora
Each film coated tablet contains:
Rosuvastatin 5mg, 10mg as Rosuvastatin calcium.
Uses
Aurora tablets are indicated:
- Primary hypercholesterolemia (type IIa including heterozygous familial hypercholesterolemia) or mixed dyslipidaemia (type IIb) as an adjunct to diet when response to diet and other non-pharmacological treatments (e.g. exercise, weight reduction) is inadequate.
- As an adjunct to diet for the treatment of patients with elevated serum TG levels (Fredrickson type IV).
- Homozygous familial hypercholesterolemia as an adjunct to diet and other lipid lowering treatments (e.g. LDL apheresis) or if such treatments are not appropriate.
Side Effect
The most frequent adverse events thought to be related to Rosuvastatin are:
- Myalgia,
- Constipation,
- Asthenia,
- Abdominal pain
- Nausea.
Body As a whole: Abdominal pain, accidental injury, chest pain, infection pain, pelvic pain and neck pain.
Cardiovascular System
Hypertension, angina pectoris, vasodilatation and palpitation.
Digestive System
Constipation, gastroenteritis, vomiting, flatulence, periodontal abscess and gastritis.
Endocrine: Diabetes mellitus.
Heroic and Lymphatic System: Anemia and ecchymosis.
Metabolic and Nutritional Disorders: Peripheral edema.
Musculoskeletal System: Arthritis, arthralgia, and pathological fracture. Nervous System
Dizziness, Insomnia, Hypertonia, Paresthesia, Depression, Anxiety, Vertigo and Neuralgia.
Respiratory System
Bronchitis, Cough increased, Dyspnea, Pneumonia, and Asthma.
Skin and Appendages
Rash and Pruritus.
Skin and Appendages: Rash and Pruritus.

Aurora 10 mg Tab
Aurora
Each film coated tablet contains:
Rosuvastatin 5mg, 10mg as Rosuvastatin calcium.
Uses
Aurora tablets are indicated:
- Primary hypercholesterolemia (type IIa including heterozygous familial hypercholesterolemia) or mixed dyslipidaemia (type IIb) as an adjunct to diet when response to diet and other non-pharmacological treatments (e.g. exercise, weight reduction) is inadequate.
- As an adjunct to diet for the treatment of patients with elevated serum TG levels (Fredrickson type IV).
- Homozygous familial hypercholesterolemia as an adjunct to diet and other lipid lowering treatments (e.g. LDL apheresis) or if such treatments are not appropriate.
Side Effect
The most frequent adverse events thought to be related to Rosuvastatin are:
- Myalgia,
- Constipation,
- Asthenia,
- Abdominal pain
- Nausea.
Body As a whole: Abdominal pain, accidental injury, chest pain, infection pain, pelvic pain and neck pain.
Cardiovascular System
Hypertension, angina pectoris, vasodilatation and palpitation.
Digestive System
Constipation, gastroenteritis, vomiting, flatulence, periodontal abscess and gastritis.
Endocrine: Diabetes mellitus.
Heroic and Lymphatic System: Anemia and ecchymosis.
Metabolic and Nutritional Disorders: Peripheral edema.
Musculoskeletal System: Arthritis, arthralgia, and pathological fracture. Nervous System
Dizziness, Insomnia, Hypertonia, Paresthesia, Depression, Anxiety, Vertigo and Neuralgia.
Respiratory System
Bronchitis, Cough increased, Dyspnea, Pneumonia, and Asthma.
Skin and Appendages
Rash and Pruritus.
Skin and Appendages: Rash and Pruritus.

Carveda 6.25 mg Tab
Carveda
Composition
Each tablet contains carvedilol 6.25 mg
Uses
Hypertension
Heart failure
Left ventricular dysfunction following myocardial infarction
Side Effect
Heart failure and left ventricular dysfunction following myocardial infarction (>10%): Dizziness, fatigue, hypotension, diarrhea, hyperglycemia, bradycardia, weight increase. Hypertension(>5%): Dizziness.
ABC Apixaban 5mg Tag
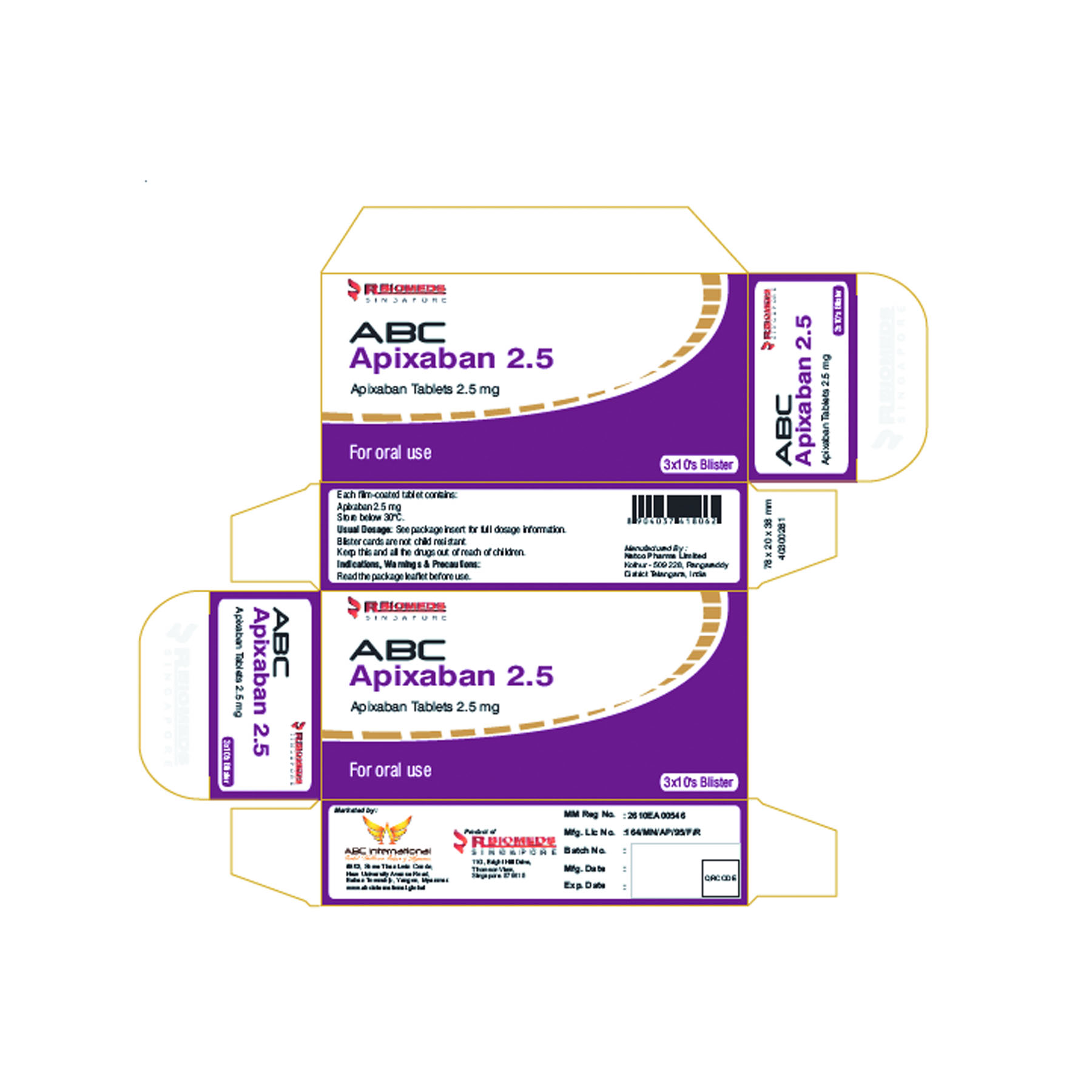
ABC Apixaban 2.5mg Tag

Atorsun 10
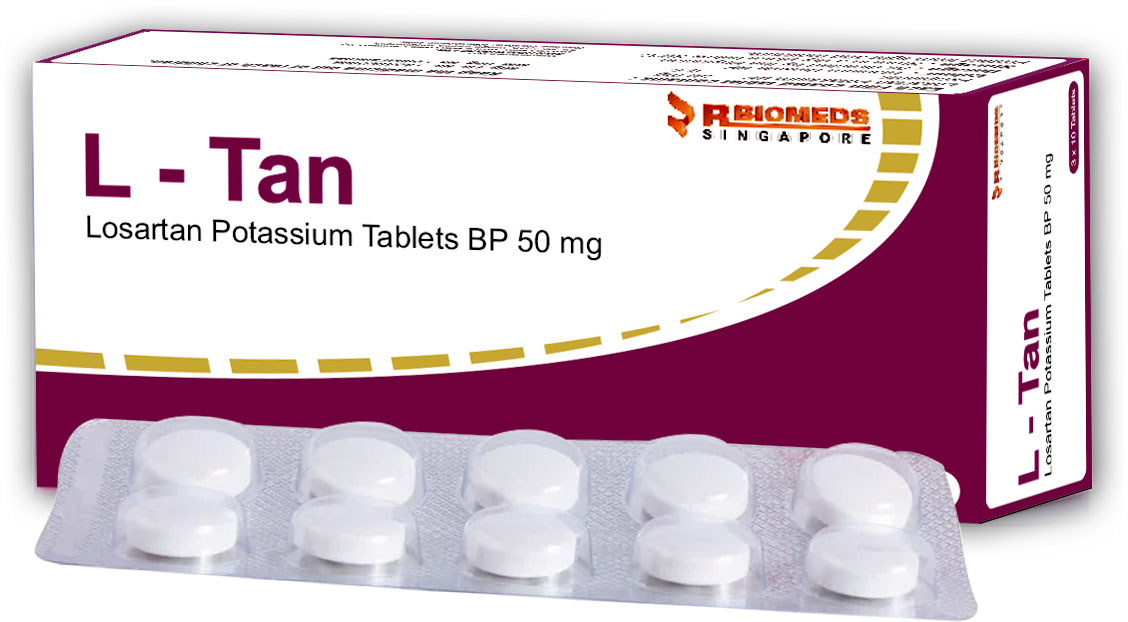
L-Tan Tab

Nicoril 10 mg Tab

Xavor 50 mg Tab
Xavor
Each film coated tablet contains:
Losartan potassium 50mg & Hydrochlorothiazide 12.5mg; Inactive Ingredients: Colloidal Anhydrous Silica; Magnesium Stearate; Sodium Starch Glycolate; Talcum; Microcrystalline Cellulose; Opadry Orange 85G53561; Carnauba Wax.
Uses
XAVOR-DIU is indicated for the treatment of hypertension.
XAVOR-DIU is indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy.
Side Effect
Edema, Palpitation, Dizziness, Abdominal pain, Back pain, cough, sinusitis
Bilirubin increased (serum), BUN increased, hematocrit decreased, hemoglobin decreased, hyper-/hypotension hyponatremia, liver enzymes increased, rhabdomyolysis, serum creatinine increased, thrombocytopenia.

Xavor DIU Tab
Xavor
Each film coated tablet contains:
Losartan potassium 50mg & Hydrochlorothiazide 12.5mg; Inactive Ingredients: Colloidal Anhydrous Silica; Magnesium Stearate; Sodium Starch Glycolate; Talcum; Microcrystalline Cellulose; Opadry Orange 85G53561; Carnauba Wax.
Uses
XAVOR-DIU is indicated for the treatment of hypertension.
XAVOR-DIU is indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy.
Side Effect
Edema, Palpitation, Dizziness, Abdominal pain, Back pain, cough, sinusitis
Bilirubin increased (serum), BUN increased, hematocrit decreased, hemoglobin decreased, hyper-/hypotension hyponatremia, liver enzymes increased, rhabdomyolysis, serum creatinine increased, thrombocytopenia.

Aurora 5 mg Tab
Aurora
Each film coated tablet contains:
Rosuvastatin 5mg, 10mg as Rosuvastatin calcium.
Uses
Aurora tablets are indicated:
- Primary hypercholesterolemia (type IIa including heterozygous familial hypercholesterolemia) or mixed dyslipidaemia (type IIb) as an adjunct to diet when response to diet and other non-pharmacological treatments (e.g. exercise, weight reduction) is inadequate.
- As an adjunct to diet for the treatment of patients with elevated serum TG levels (Fredrickson type IV).
- Homozygous familial hypercholesterolemia as an adjunct to diet and other lipid lowering treatments (e.g. LDL apheresis) or if such treatments are not appropriate.
Side Effect
The most frequent adverse events thought to be related to Rosuvastatin are:
- Myalgia,
- Constipation,
- Asthenia,
- Abdominal pain
- Nausea.
Body As a whole: Abdominal pain, accidental injury, chest pain, infection pain, pelvic pain and neck pain.
Cardiovascular System
Hypertension, angina pectoris, vasodilatation and palpitation.
Digestive System
Constipation, gastroenteritis, vomiting, flatulence, periodontal abscess and gastritis.
Endocrine: Diabetes mellitus.
Heroic and Lymphatic System: Anemia and ecchymosis.
Metabolic and Nutritional Disorders: Peripheral edema.
Musculoskeletal System: Arthritis, arthralgia, and pathological fracture. Nervous System
Dizziness, Insomnia, Hypertonia, Paresthesia, Depression, Anxiety, Vertigo and Neuralgia.
Respiratory System
Bronchitis, Cough increased, Dyspnea, Pneumonia, and Asthma.
Skin and Appendages
Rash and Pruritus.
Skin and Appendages: Rash and Pruritus.

Aurora 10 mg Tab
Aurora
Each film coated tablet contains:
Rosuvastatin 5mg, 10mg as Rosuvastatin calcium.
Uses
Aurora tablets are indicated:
- Primary hypercholesterolemia (type IIa including heterozygous familial hypercholesterolemia) or mixed dyslipidaemia (type IIb) as an adjunct to diet when response to diet and other non-pharmacological treatments (e.g. exercise, weight reduction) is inadequate.
- As an adjunct to diet for the treatment of patients with elevated serum TG levels (Fredrickson type IV).
- Homozygous familial hypercholesterolemia as an adjunct to diet and other lipid lowering treatments (e.g. LDL apheresis) or if such treatments are not appropriate.
Side Effect
The most frequent adverse events thought to be related to Rosuvastatin are:
- Myalgia,
- Constipation,
- Asthenia,
- Abdominal pain
- Nausea.
Body As a whole: Abdominal pain, accidental injury, chest pain, infection pain, pelvic pain and neck pain.
Cardiovascular System
Hypertension, angina pectoris, vasodilatation and palpitation.
Digestive System
Constipation, gastroenteritis, vomiting, flatulence, periodontal abscess and gastritis.
Endocrine: Diabetes mellitus.
Heroic and Lymphatic System: Anemia and ecchymosis.
Metabolic and Nutritional Disorders: Peripheral edema.
Musculoskeletal System: Arthritis, arthralgia, and pathological fracture. Nervous System
Dizziness, Insomnia, Hypertonia, Paresthesia, Depression, Anxiety, Vertigo and Neuralgia.
Respiratory System
Bronchitis, Cough increased, Dyspnea, Pneumonia, and Asthma.
Skin and Appendages
Rash and Pruritus.
Skin and Appendages: Rash and Pruritus.

Carveda 6.25 mg Tab
Carveda
Composition
Each tablet contains carvedilol 6.25 mg
Uses
Hypertension
Heart failure
Left ventricular dysfunction following myocardial infarction
Side Effect
Heart failure and left ventricular dysfunction following myocardial infarction (>10%): Dizziness, fatigue, hypotension, diarrhea, hyperglycemia, bradycardia, weight increase. Hypertension(>5%): Dizziness.

ABC Apixaban 5mg Tag

ABC Apixaban 2.5mg Tag

Atorsun 10

L-Tan Tab

Nicoril 10 mg Tab

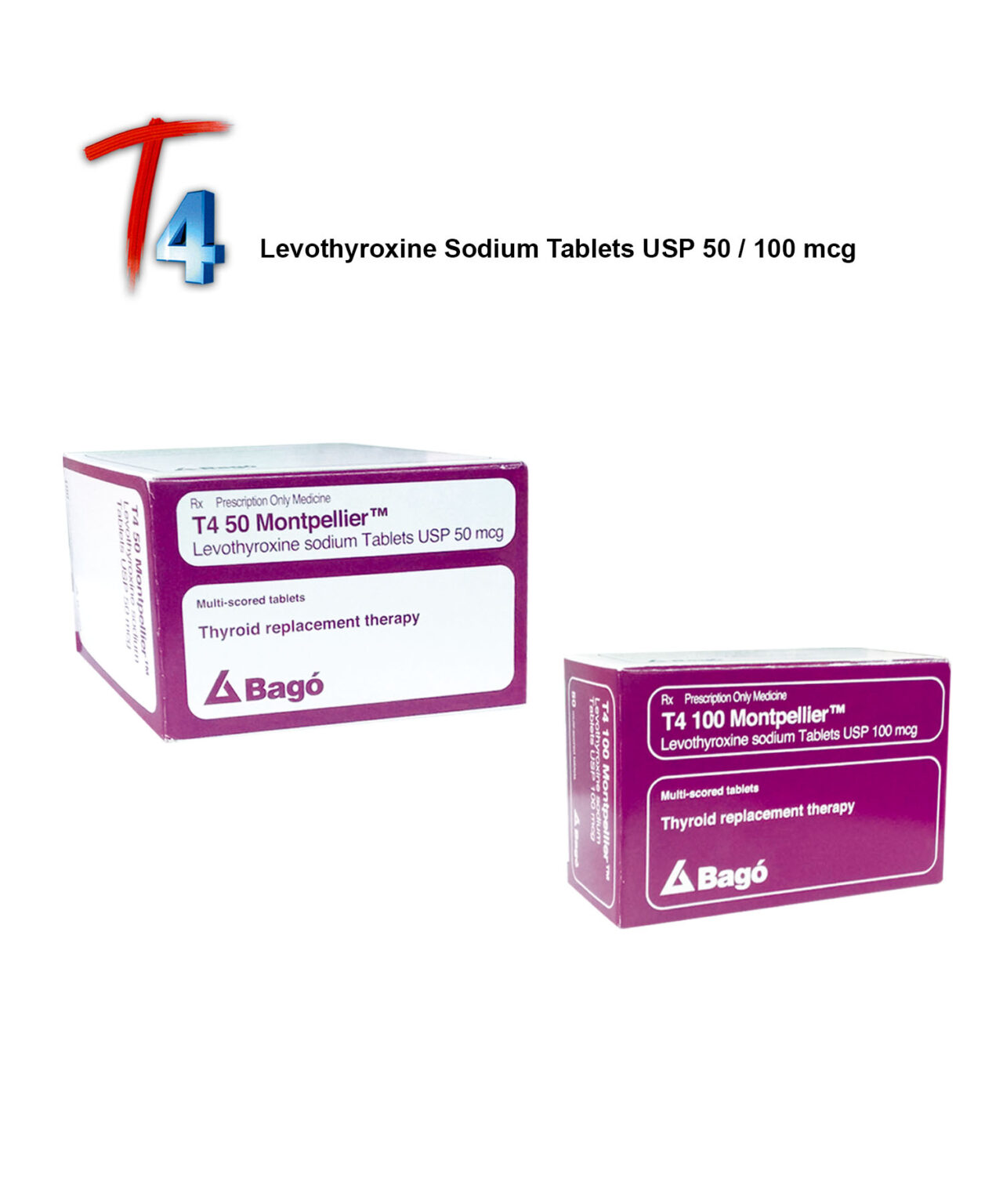
T4 50 mcg Tab
T4 50 mcg/ 100 mcg Tab
Each multi-scored tablet contains
LEVOTHYROXINE SODIUM 50 mcg/ 100 mcg
Inactive Ingredients:
Lactose, Microcrystalline Cellulose, Cellulose powder, Croscaramellose sodium, Magnesium Stearate, Quinoline yellow ( aluminium lake)
Uses
- As replacement or supplement therapy in patients with hypothyroidism of any aetiology (except transient hypothyroidism during the recovery phase of sub-acute thyroiditis): primary hypothyroidism resulting from functional deficiency, primary atrophy, partial or total congenital absence of the thyroid gland, or from the effects of surgery, radiation or drugs, with or without the presence of goitre; secondary (pituitary)
- hypothyroidism; and tertiary (hypothalamic) hypothyroidism and subclinical hypothyroidism.
- For the suppression of pituitary Thyrotropin (TSH) in the treatment or prevention of various types of euthyroid goitres, including thyroid nodules, sub-acute or chronic lymphocytic thyroiditis (Hashimoto’s thyroiditis), multinodular goitre and as an adjunct to surgery and radiolabelled iodine therapy in the management of TSH-dependent thyroid papillary carcinoma or well differentiated follicular carcinoma.
Side Effect
Adverse reactions different from those indicative of thyrotoxicosis as a result of therapeutic overdosage (either initially or during maintenance) are rare.In infants receiving hormone replacement therapy acraniosynostosis has been associated to latrogenic hyperthyroidism.
Inadequate dosage of the product may fail to resolve hypothyroidism symptoms.
There may be hypersensitivity reactions, such as rash and urticaria, to inactive ingredients in the product.
There may be partial hair loss during the early months of therapy, although this is generally transient.
Benign intracranial hypertension has been reported in children receiving levothyroxine therapy.

T4 100 mcg Tab
T4 50 mcg/ 100 mcg Tab
Each multi-scored tablet contains
LEVOTHYROXINE SODIUM 50 mcg/ 100 mcg
Inactive Ingredients:
Lactose, Microcrystalline Cellulose, Cellulose powder, Croscaramellose sodium, Magnesium Stearate, Quinoline yellow ( aluminium lake)
Uses
- As replacement or supplement therapy in patients with hypothyroidism of any aetiology (except transient hypothyroidism during the recovery phase of sub-acute thyroiditis): primary hypothyroidism resulting from functional deficiency, primary atrophy, partial or total congenital absence of the thyroid gland, or from the effects of surgery, radiation or drugs, with or without the presence of goitre; secondary (pituitary)
- hypothyroidism; and tertiary (hypothalamic) hypothyroidism and subclinical hypothyroidism.
- For the suppression of pituitary Thyrotropin (TSH) in the treatment or prevention of various types of euthyroid goitres, including thyroid nodules, sub-acute or chronic lymphocytic thyroiditis (Hashimoto’s thyroiditis), multinodular goitre and as an adjunct to surgery and radiolabelled iodine therapy in the management of TSH-dependent thyroid papillary carcinoma or well differentiated follicular carcinoma.
Side Effect
Adverse reactions different from those indicative of thyrotoxicosis as a result of therapeutic overdosage (either initially or during maintenance) are rare.In infants receiving hormone replacement therapy acraniosynostosis has been associated to latrogenic hyperthyroidism.
Inadequate dosage of the product may fail to resolve hypothyroidism symptoms.
There may be hypersensitivity reactions, such as rash and urticaria, to inactive ingredients in the product.
There may be partial hair loss during the early months of therapy, although this is generally transient.
Benign intracranial hypertension has been reported in children receiving levothyroxine therapy.
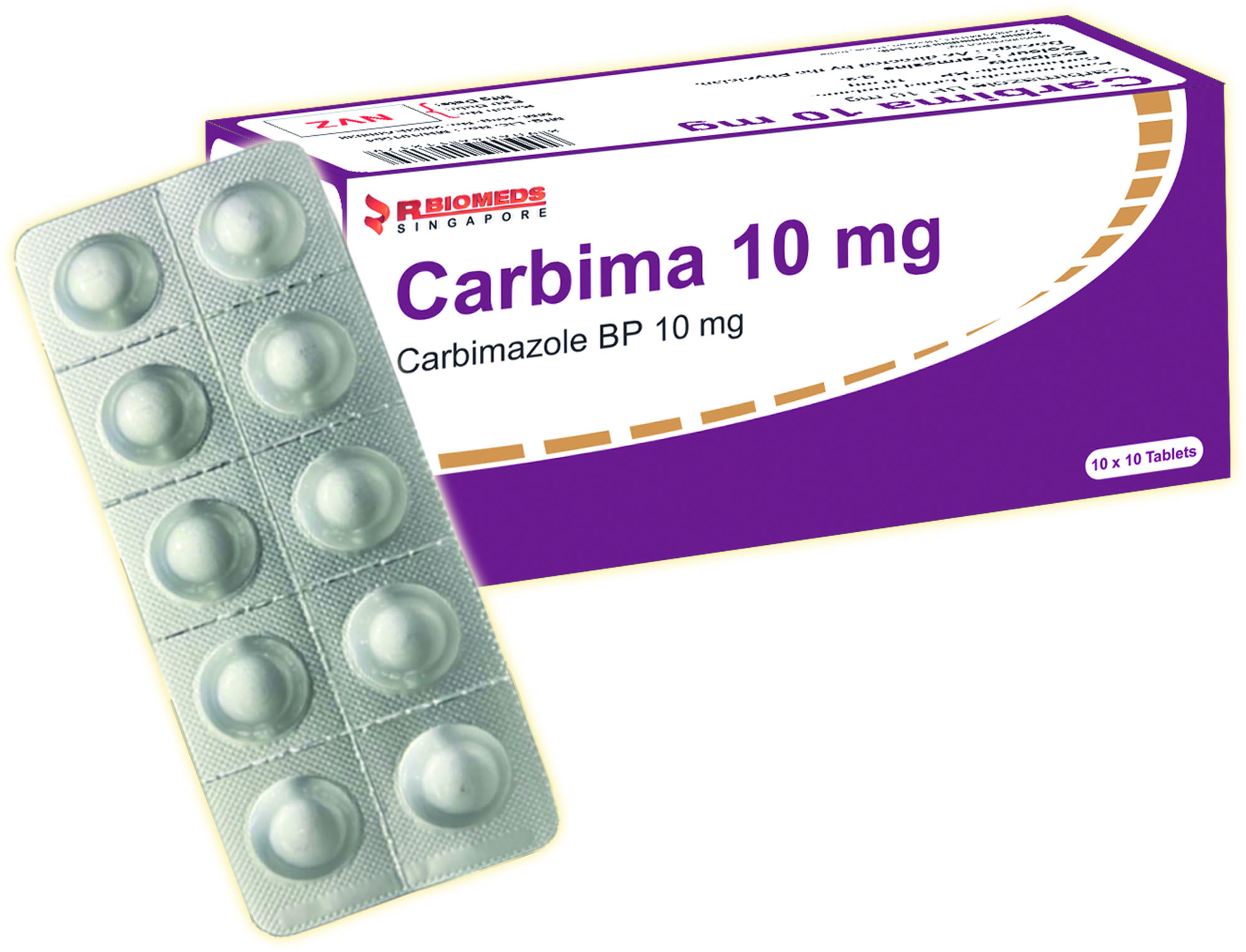
Carbima 10mg

Glemaz 4mg
Thyro-Med 50mcg

Thyro-Med 100mcg

T4 50 mcg Tab
T4 50 mcg/ 100 mcg Tab
Each multi-scored tablet contains
LEVOTHYROXINE SODIUM 50 mcg/ 100 mcg
Inactive Ingredients:
Lactose, Microcrystalline Cellulose, Cellulose powder, Croscaramellose sodium, Magnesium Stearate, Quinoline yellow ( aluminium lake)
Uses
- As replacement or supplement therapy in patients with hypothyroidism of any aetiology (except transient hypothyroidism during the recovery phase of sub-acute thyroiditis): primary hypothyroidism resulting from functional deficiency, primary atrophy, partial or total congenital absence of the thyroid gland, or from the effects of surgery, radiation or drugs, with or without the presence of goitre; secondary (pituitary)
- hypothyroidism; and tertiary (hypothalamic) hypothyroidism and subclinical hypothyroidism.
- For the suppression of pituitary Thyrotropin (TSH) in the treatment or prevention of various types of euthyroid goitres, including thyroid nodules, sub-acute or chronic lymphocytic thyroiditis (Hashimoto’s thyroiditis), multinodular goitre and as an adjunct to surgery and radiolabelled iodine therapy in the management of TSH-dependent thyroid papillary carcinoma or well differentiated follicular carcinoma.
Side Effect
Adverse reactions different from those indicative of thyrotoxicosis as a result of therapeutic overdosage (either initially or during maintenance) are rare.In infants receiving hormone replacement therapy acraniosynostosis has been associated to latrogenic hyperthyroidism.
Inadequate dosage of the product may fail to resolve hypothyroidism symptoms.
There may be hypersensitivity reactions, such as rash and urticaria, to inactive ingredients in the product.
There may be partial hair loss during the early months of therapy, although this is generally transient.
Benign intracranial hypertension has been reported in children receiving levothyroxine therapy.

T4 100 mcg Tab
T4 50 mcg/ 100 mcg Tab
Each multi-scored tablet contains
LEVOTHYROXINE SODIUM 50 mcg/ 100 mcg
Inactive Ingredients:
Lactose, Microcrystalline Cellulose, Cellulose powder, Croscaramellose sodium, Magnesium Stearate, Quinoline yellow ( aluminium lake)
Uses
- As replacement or supplement therapy in patients with hypothyroidism of any aetiology (except transient hypothyroidism during the recovery phase of sub-acute thyroiditis): primary hypothyroidism resulting from functional deficiency, primary atrophy, partial or total congenital absence of the thyroid gland, or from the effects of surgery, radiation or drugs, with or without the presence of goitre; secondary (pituitary)
- hypothyroidism; and tertiary (hypothalamic) hypothyroidism and subclinical hypothyroidism.
- For the suppression of pituitary Thyrotropin (TSH) in the treatment or prevention of various types of euthyroid goitres, including thyroid nodules, sub-acute or chronic lymphocytic thyroiditis (Hashimoto’s thyroiditis), multinodular goitre and as an adjunct to surgery and radiolabelled iodine therapy in the management of TSH-dependent thyroid papillary carcinoma or well differentiated follicular carcinoma.
Side Effect
Adverse reactions different from those indicative of thyrotoxicosis as a result of therapeutic overdosage (either initially or during maintenance) are rare.In infants receiving hormone replacement therapy acraniosynostosis has been associated to latrogenic hyperthyroidism.
Inadequate dosage of the product may fail to resolve hypothyroidism symptoms.
There may be hypersensitivity reactions, such as rash and urticaria, to inactive ingredients in the product.
There may be partial hair loss during the early months of therapy, although this is generally transient.
Benign intracranial hypertension has been reported in children receiving levothyroxine therapy.

Carbima 10mg

Glemaz 4mg

Thyro-Med 50mcg

Thyro-Med 100mcg


Carbomark 450 mg Inj
Carbomark
Composition
Carboplatin 150/450mg
Uses
Carboplatin is used to treat a number of Cancers which include ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma, testicular cancer and triple-negative breast cancer.
Side Effect
Common side effects of carboplatin include:
nausea, vomiting, numbness and tingling of extremities, ear infection, pain, weakness, allergic reactions, and hair loss.
Serious side effects of carboplatin include: bleeding and reduced blood cells, including reduced red blood cells (anemia) and platelets (needed for proper blood clotting);
unusual bruising or bleeding, black tarry stools or blood in the urine;
infection;
life-threatening allergic reaction;
kidney and liver problems; or
loss of hearing or ringing in the ears.

Carbomark 150 mg In
Carbomark
Composition
Carboplatin 150/450mg
Uses
Carboplatin is used to treat a number of Cancers which include ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma, testicular cancer and triple-negative breast cancer.
Side Effect
Common side effects of carboplatin include:
nausea, vomiting, numbness and tingling of extremities, ear infection, pain, weakness, allergic reactions, and hair loss.
Serious side effects of carboplatin include: bleeding and reduced blood cells, including reduced red blood cells (anemia) and platelets (needed for proper blood clotting);
unusual bruising or bleeding, black tarry stools or blood in the urine;
infection;
life-threatening allergic reaction;
kidney and liver problems; or
loss of hearing or ringing in the ears.

Cismark 50 mg Inj
Cismark
Composition
Cisplatin 50mg
Uses
Cisplatin is used for the treatment of advanced ovarian cancer, testicular cancer, and bladder carcinoma
Side Effect
Nausea, Vomiting, Diarrhea, Temporary hair loss, Loss in ability to taste food, Hiccups, Dry mouth, Dark urine, Decreased sweating, Dry skin , Signs of dehydration, Rash, Itching, Blurred vision, Dizziness, Drowsiness, Headache, Allergic reactions, Blisters, Redness of skin, Peeling of the skin, Restlessness, Lightheadedness, Feeling exhausted too early, Stomach pain, Abdominal pain, Body pain, Swollen legs, Face swelling, Swollen throat, Numbness, Reduce in the number of white blood cells, Bruising and leeding, Anaemia (low number of red blood cells), Feeling sick, Loss of appetite, hearing loss.

Doxomark 10 mg Inj
Doxomark
Composition
Doxorubicin 10/50mg
Uses
Doxorubicin is commonly used to treat some leukemias and Hodgkin’s lymphoma, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma.
Side Effect
Cough or hoarseness accompanied by fever or chills
darkening or redness of the skin (if you recently had radiation treatment)
fast or irregular heartbeat
fever or chills
joint pain
lower back or side pain accompanied by fever or chills
pain at the injection site
painful or difficult urination accompanied by fever or chills
red streaks along the injected vein
shortness of breath
stomach pain
swelling of the feet and lower legs
Rare
Black, tarry stools
blood in the urine
pinpoint red spots on the skin
unusual bleeding or bruising.

Doxomark 50 mg Inj
Doxomark
Composition
Doxorubicin 10/50mg
Uses
Doxorubicin is commonly used to treat some leukemias and Hodgkin’s lymphoma, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma.
Side Effect
Cough or hoarseness accompanied by fever or chills
darkening or redness of the skin (if you recently had radiation treatment)
fast or irregular heartbeat
fever or chills
joint pain
lower back or side pain accompanied by fever or chills
pain at the injection site
painful or difficult urination accompanied by fever or chills
red streaks along the injected vein
shortness of breath
stomach pain
swelling of the feet and lower legs
Rare
Black, tarry stools
blood in the urine
pinpoint red spots on the skin
unusual bleeding or bruising.

Gemcel 1000 mg Inj
Gemcel
Composition
Gemcitabine 200mg/ 1gm
Uses
Pancreatic Cancer, Non-small Cell Lung Cancer,
Breast Cancer,
Ovarian Cancer
Side Effect
Anemia (65%), Elev LFTs (68%), Neutropenia (63%), Leukopenia (62%), Pain (48%), Proteinuria (45%), Fever (41%), Hematuria (35%), Rash (30%), Thrombocytopenia (24%), Dyspnea (23%), Constipation (23%), Diarrhea (19%), Flu-like syndrome (19%), Hemorrhage (17%), BUN increased (16%), Infection (16%), Alopecia (15%), Edema (13%), Elev bilirubin (13%), 1-10%, Paresthesia (2-10%), Creatinine increased (2-8%), Inj site reactions (4%), Bronchospasm (2%)
Postmarketing Reports
Cardiovascular: CHF, MI, arrhythmias, supraventricular arrhythmias
Vascular disorders: Peripheral vasculitis, gangrene, capillary leak syndrome
Skin: Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
Hepatic: Hepatic failure, hepatic veno-occlusive disease
Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, ARDS
Pulmonary: Pulmonary eosinophilia
Blood and lymphatic system: Thrombotic microangiopathy

Gemcel 200 mg Inj
Gemcel
Composition
Gemcitabine 200mg/ 1gm
Uses
Pancreatic Cancer, Non-small Cell Lung Cancer,
Breast Cancer,
Ovarian Cancer
Side Effect
Anemia (65%), Elev LFTs (68%), Neutropenia (63%), Leukopenia (62%), Pain (48%), Proteinuria (45%), Fever (41%), Hematuria (35%), Rash (30%), Thrombocytopenia (24%), Dyspnea (23%), Constipation (23%), Diarrhea (19%), Flu-like syndrome (19%), Hemorrhage (17%), BUN increased (16%), Infection (16%), Alopecia (15%), Edema (13%), Elev bilirubin (13%), 1-10%, Paresthesia (2-10%), Creatinine increased (2-8%), Inj site reactions (4%), Bronchospasm (2%)
Postmarketing Reports
Cardiovascular: CHF, MI, arrhythmias, supraventricular arrhythmias
Vascular disorders: Peripheral vasculitis, gangrene, capillary leak syndrome
Skin: Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
Hepatic: Hepatic failure, hepatic veno-occlusive disease
Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, ARDS
Pulmonary: Pulmonary eosinophilia
Blood and lymphatic system: Thrombotic microangiopathy

Oxamark 50 mg Inj
Oxamark
Composition
Oxaliplatin 50/100mg
Uses
For management of Colorectal Cancers
Side Effect
Peripheral neuropathy (76%), Anemia (64%), Nausea (64%), Fatigue (61%), Diarrhea (46%), Vomiting (37%), Abdominal pain (31%), Constipation(31%), Thrombocytopenia (30%), Fever (25%), Anorexia (20%), Leukopenia (13%), Dyspnea (13%), Cough (11%), 1-10%, Edema (10%), Neutropenia (7%), Pharyngolaryngeal dysesthesia (1-2%), <1%, Pulmonary fibrosis, Posterior leukoencephalopathy syndrome.
pathy

Oxamark 100 mg Inj
Oxamark
Composition
Oxaliplatin 50/100mg
Uses
For management of Colorectal Cancers
Side Effect
Peripheral neuropathy (76%), Anemia (64%), Nausea (64%), Fatigue (61%), Diarrhea (46%), Vomiting (37%), Abdominal pain (31%), Constipation(31%), Thrombocytopenia (30%), Fever (25%), Anorexia (20%), Leukopenia (13%), Dyspnea (13%), Cough (11%), 1-10%, Edema (10%), Neutropenia (7%), Pharyngolaryngeal dysesthesia (1-2%), <1%, Pulmonary fibrosis, Posterior leukoencephalopathy syndrome.
pathy

Paclimark 30 mg Inj
Paclimark
Composition
Paclitaxel 30/100/260mg
Novapressin contains
Uses
Breast cancer, Ovarian cancer, Non-Small cell Lung cancer, AIDS related Kaposi’s Sarcoma
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
Neutropenia (78-100%), Alopecia (55-96%), Anemia (47-96%), Arthralgia/myalgia (93%), Diarrhea (90%), Leukopenia (90%), Nausea/vomiting (9-88%), Opportunistic infections (76%), Peripheral neuropathy (42-79%), Thrombocytopenia (4-68%), Mucositis (5-45%), Hypersensitivity (2-45%), Renal impairment (34%), Hypotension (17%), 1-10%, Bradycardia (3%), <1%, Grand mal seizures, Cardiac conduction abnormalities.
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Paclimark 100 mg Inj
Paclimark
Composition
Paclitaxel 30/100/260mg
Novapressin contains
Uses
Breast cancer, Ovarian cancer, Non-Small cell Lung cancer, AIDS related Kaposi’s Sarcoma
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
Neutropenia (78-100%), Alopecia (55-96%), Anemia (47-96%), Arthralgia/myalgia (93%), Diarrhea (90%), Leukopenia (90%), Nausea/vomiting (9-88%), Opportunistic infections (76%), Peripheral neuropathy (42-79%), Thrombocytopenia (4-68%), Mucositis (5-45%), Hypersensitivity (2-45%), Renal impairment (34%), Hypotension (17%), 1-10%, Bradycardia (3%), <1%, Grand mal seizures, Cardiac conduction abnormalities.
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Paclimark 260 mg Inj
Paclimark
Composition
Paclitaxel 30/100/260mg
Novapressin contains
Uses
Breast cancer, Ovarian cancer, Non-Small cell Lung cancer, AIDS related Kaposi’s Sarcoma
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
Neutropenia (78-100%), Alopecia (55-96%), Anemia (47-96%), Arthralgia/myalgia (93%), Diarrhea (90%), Leukopenia (90%), Nausea/vomiting (9-88%), Opportunistic infections (76%), Peripheral neuropathy (42-79%), Thrombocytopenia (4-68%), Mucositis (5-45%), Hypersensitivity (2-45%), Renal impairment (34%), Hypotension (17%), 1-10%, Bradycardia (3%), <1%, Grand mal seizures, Cardiac conduction abnormalities.
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Donataxel 20 mg Inj
Donataxel
Composition
Docetaxel 20/80mg
Uses
Breast Cancer, Prostate cancer, Non-Small cell Lung cancer
Side Effect
>50% Alopecia, Anemia, Leukopenia, Neutropenia, Asthenia, 10-50%, Fever, Infections, Fluid retention, Hypersensitivity, Skin reactions, Diarrhea, Nausea, Vomiting, Sensory neuropathy, Myalgia, Nail changes, 1-10%, Arthralgia, Thrombocytopenia.

Donataxel 80 mg Inj
Donataxel
Composition
Docetaxel 20/80mg
Uses
Breast Cancer, Prostate cancer, Non-Small cell Lung cancer
Side Effect
>50% Alopecia, Anemia, Leukopenia, Neutropenia, Asthenia, 10-50%, Fever, Infections, Fluid retention, Hypersensitivity, Skin reactions, Diarrhea, Nausea, Vomiting, Sensory neuropathy, Myalgia, Nail changes, 1-10%, Arthralgia, Thrombocytopenia.
Doxite 50 mcg Inj
Doxtie
Composition
Doxorubicin 50mg
Uses
Doxorubicin is commonly used to treat some leukemias and Hodgkin’s lymphoma, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma.
Side Effect
Cough or hoarseness accompanied by fever or chills
darkening or redness of the skin (if you recently had radiation treatment)
fast or irregular heartbeat
fever or chills
joint pain
lower back or side pain accompanied by fever or chills
pain at the injection site
painful or difficult urination accompanied by fever or chills
red streaks along the injected vein
shortness of breath
stomach pain
swelling of the feet and lower legs
Rare
Black, tarry stools
blood in the urine
pinpoint red spots on the skin
unusual bleeding or bruising.
Gembio 200 mg Inj
Gembio
Composition
Gemcitabine 200mg/ 1gm
Uses
Pancreatic Cancer, Non-small Cell Lung Cancer,
Breast Cancer,
Ovarian Cancer
Side Effect
Anemia (65%), Elev LFTs (68%), Neutropenia (63%), Leukopenia (62%), Pain (48%), Proteinuria (45%), Fever (41%), Hematuria (35%), Rash (30%), Thrombocytopenia (24%), Dyspnea (23%), Constipation (23%), Diarrhea (19%), Flu-like syndrome (19%), Hemorrhage (17%), BUN increased (16%), Infection (16%), Alopecia (15%), Edema (13%), Elev bilirubin (13%), 1-10%, Paresthesia (2-10%), Creatinine increased (2-8%), Inj site reactions (4%), Bronchospasm (2%)
Postmarketing Reports
Cardiovascular: CHF, MI, arrhythmias, supraventricular arrhythmias
Vascular disorders: Peripheral vasculitis, gangrene, capillary leak syndrome
Skin: Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
Hepatic: Hepatic failure, hepatic veno-occlusive disease
Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, ARDS
Pulmonary: Pulmonary eosinophilia
Blood and lymphatic system: Thrombotic microangiopat

Gembio 1 gm Inj
Gembio
Composition
Gemcitabine 200mg/ 1gm
Uses
Pancreatic Cancer, Non-small Cell Lung Cancer,
Breast Cancer,
Ovarian Cancer
Side Effect
Anemia (65%), Elev LFTs (68%), Neutropenia (63%), Leukopenia (62%), Pain (48%), Proteinuria (45%), Fever (41%), Hematuria (35%), Rash (30%), Thrombocytopenia (24%), Dyspnea (23%), Constipation (23%), Diarrhea (19%), Flu-like syndrome (19%), Hemorrhage (17%), BUN increased (16%), Infection (16%), Alopecia (15%), Edema (13%), Elev bilirubin (13%), 1-10%, Paresthesia (2-10%), Creatinine increased (2-8%), Inj site reactions (4%), Bronchospasm (2%)
Postmarketing Reports
Cardiovascular: CHF, MI, arrhythmias, supraventricular arrhythmias
Vascular disorders: Peripheral vasculitis, gangrene, capillary leak syndrome
Skin: Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
Hepatic: Hepatic failure, hepatic veno-occlusive disease
Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, ARDS
Pulmonary: Pulmonary eosinophilia
Blood and lymphatic system: Thrombotic microangiopat

Oncofusion IV Infusion Set
Oncofusion
Uses
PVC free IV set for administration of drugs which can react with PVC in normal IV sets (eg: Paclitaxel, Docetaxel etc)

Tzmide 20 mg Inj
TZmide Capsule
Composition
Temozopamide 20/100mg. Capsule
Uses
Astrocytoma, Glioblastoma Multiforme
Side Effect
Alopecia (55-69%), Lymphopenia (55%), Nausea (53%), Vomiting (42%), Headache (41%), Fatigue (34%), Constipation (33%), Anorexia (9-27%), Convulsions (23%), Thrombocytopenia (19%), Rash (8-19%), Hemiparesis (18%), Diarrhea (16%), Neutropenia (14%), Fever (13%), Asthenia (13%), Dizziness (12%), Peripheral edema (11%), Viral infections (11%), 1-10% (selected), Amnesia (10%), Insomnia (10%), Abdominal pain (5-9%), Ataxia (8%), Back pain (8%), Paresis (8%), URI (8%), Urinary incontinence (8%), UTI (8%), Abnormal vision (5-8%), Pruritus (5-8%), Breast pain (6%), Depression (6%), Confusion (5%), Myalgia (5%), Weight gain (5%), Anemia (4%), Erythema (1%).

Tzmide 100 mg Inj
TZmide Capsule
Composition
Temozopamide 20/100mg. Capsule
Uses
Astrocytoma, Glioblastoma Multiforme
Side Effect
Alopecia (55-69%), Lymphopenia (55%), Nausea (53%), Vomiting (42%), Headache (41%), Fatigue (34%), Constipation (33%), Anorexia (9-27%), Convulsions (23%), Thrombocytopenia (19%), Rash (8-19%), Hemiparesis (18%), Diarrhea (16%), Neutropenia (14%), Fever (13%), Asthenia (13%), Dizziness (12%), Peripheral edema (11%), Viral infections (11%), 1-10% (selected), Amnesia (10%), Insomnia (10%), Abdominal pain (5-9%), Ataxia (8%), Back pain (8%), Paresis (8%), URI (8%), Urinary incontinence (8%), UTI (8%), Abnormal vision (5-8%), Pruritus (5-8%), Breast pain (6%), Depression (6%), Confusion (5%), Myalgia (5%), Weight gain (5%), Anemia (4%), Erythema (1%).

Bortemark 3.5 mg Inj
Bortemark
Composition
Bortezomib 3.5mg
Uses
Mantle cell Lymphoma, Multiple myeloma
Side Effect
Asthenia (61-65%), Nausea (61-65%), Diarrhea (51-55%), Anorexia (41-45%), Constipation (41-45%), Thrombocytopenia (41-45%), Peripheral neuropathy (IV: 16-41%; SC: 6-24%), Pyrexia (36-40%), Vomiting (36-40%), Anemia (31-35%), Arthralgia (26-30%), Headache (26-30%), Insomnia (26-30%), Limb pain (26-30%), Dizziness (21-25%), Dyspnea (21-25%), Edema (21-25%), Neutropenia (21-25%), Paresthesia (21-25%), Rash (21-25%), Cough (15-20%), Dehydration (15-20%), URI (15-20%), Rigors, grade 4 toxicity (10-15%), Frequency Not Defined, Hypotension, Anxiety, Pain, Pruritus, Abdominal pain, Dyspepsia, Back pain, Bone pain, Myalgia, Muscle spasms, Herpes zoster, Pneumonia, Blurred vision.

Photofusion IV Infusion Set
Photofusion
Uses
For administration of Photosensitive (light sensitive) drugs
Idelara 2.5 mg
IDelara
Composition
Letrozole 2.5mg tablets
Uses
Breast Cancer in Postmenopausal women, Hornone receptor Positive Breast Cancer
Side Effect
Diaphoresis (24%), Bone pain (22%), Hot flashes (19%), Back pain (18%), Dyspnea (18%), Nausea (17%), Night sweats (14%), Cough (13%), Fatigue (13%), 1-10%, Constipation (10%), Hypertension (8%), Chest pain (8%), Diarrhea (8%), Decr wt (7%), Edema (7%), Breast pain (7%), Bone fractures (6%), UTI (6%), Hypercalcemia (5%), Headache (4%), Weakness (4%), Vomiting (3%), Osteoporosis (2%).

Panataxel 30 mg
Panataxel
Novapressin contains
Terlipressin Acetate (triglycyl¬lysine-vasopressin) 1mg
Uses
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Panataxel 100 mg
Panataxel
Novapressin contains
Terlipressin Acetate (triglycyl¬lysine-vasopressin) 1mg
Uses
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia
Anastronat Tablets 1 mg

Cyclophos-Med 200 mg

Cyclophos-Med 500 mg
Benzimir 25mg

Benzimir 100mg

Bevaas 100mg / 4ml

Bevaas 400mg / 16ml

Sukuba Inj
Bagocap 500mg
Tamoxis

Carbomark 450 mg Inj
Carbomark
Composition
Carboplatin 150/450mg
Uses
Carboplatin is used to treat a number of Cancers which include ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma, testicular cancer and triple-negative breast cancer.
Side Effect
Common side effects of carboplatin include:
nausea, vomiting, numbness and tingling of extremities, ear infection, pain, weakness, allergic reactions, and hair loss.
Serious side effects of carboplatin include: bleeding and reduced blood cells, including reduced red blood cells (anemia) and platelets (needed for proper blood clotting);
unusual bruising or bleeding, black tarry stools or blood in the urine;
infection;
life-threatening allergic reaction;
kidney and liver problems; or
loss of hearing or ringing in the ears.

Carbomark 150 mg In
Carbomark
Composition
Carboplatin 150/450mg
Uses
Carboplatin is used to treat a number of Cancers which include ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma, testicular cancer and triple-negative breast cancer.
Side Effect
Common side effects of carboplatin include:
nausea, vomiting, numbness and tingling of extremities, ear infection, pain, weakness, allergic reactions, and hair loss.
Serious side effects of carboplatin include: bleeding and reduced blood cells, including reduced red blood cells (anemia) and platelets (needed for proper blood clotting);
unusual bruising or bleeding, black tarry stools or blood in the urine;
infection;
life-threatening allergic reaction;
kidney and liver problems; or
loss of hearing or ringing in the ears.

Cismark 50 mg Inj
Cismark
Composition
Cisplatin 50mg
Uses
Cisplatin is used for the treatment of advanced ovarian cancer, testicular cancer, and bladder carcinoma
Side Effect
Nausea, Vomiting, Diarrhea, Temporary hair loss, Loss in ability to taste food, Hiccups, Dry mouth, Dark urine, Decreased sweating, Dry skin , Signs of dehydration, Rash, Itching, Blurred vision, Dizziness, Drowsiness, Headache, Allergic reactions, Blisters, Redness of skin, Peeling of the skin, Restlessness, Lightheadedness, Feeling exhausted too early, Stomach pain, Abdominal pain, Body pain, Swollen legs, Face swelling, Swollen throat, Numbness, Reduce in the number of white blood cells, Bruising and leeding, Anaemia (low number of red blood cells), Feeling sick, Loss of appetite, hearing loss.

Doxomark 10 mg Inj
Doxomark
Composition
Doxorubicin 10/50mg
Uses
Doxorubicin is commonly used to treat some leukemias and Hodgkin’s lymphoma, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma.
Side Effect
Cough or hoarseness accompanied by fever or chills
darkening or redness of the skin (if you recently had radiation treatment)
fast or irregular heartbeat
fever or chills
joint pain
lower back or side pain accompanied by fever or chills
pain at the injection site
painful or difficult urination accompanied by fever or chills
red streaks along the injected vein
shortness of breath
stomach pain
swelling of the feet and lower legs
Rare
Black, tarry stools
blood in the urine
pinpoint red spots on the skin
unusual bleeding or bruising.

Doxomark 50 mg Inj
Doxomark
Composition
Doxorubicin 10/50mg
Uses
Doxorubicin is commonly used to treat some leukemias and Hodgkin’s lymphoma, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma.
Side Effect
Cough or hoarseness accompanied by fever or chills
darkening or redness of the skin (if you recently had radiation treatment)
fast or irregular heartbeat
fever or chills
joint pain
lower back or side pain accompanied by fever or chills
pain at the injection site
painful or difficult urination accompanied by fever or chills
red streaks along the injected vein
shortness of breath
stomach pain
swelling of the feet and lower legs
Rare
Black, tarry stools
blood in the urine
pinpoint red spots on the skin
unusual bleeding or bruising.

Gemcel 1000 mg Inj
Gemcel
Composition
Gemcitabine 200mg/ 1gm
Uses
Pancreatic Cancer, Non-small Cell Lung Cancer,
Breast Cancer,
Ovarian Cancer
Side Effect
Anemia (65%), Elev LFTs (68%), Neutropenia (63%), Leukopenia (62%), Pain (48%), Proteinuria (45%), Fever (41%), Hematuria (35%), Rash (30%), Thrombocytopenia (24%), Dyspnea (23%), Constipation (23%), Diarrhea (19%), Flu-like syndrome (19%), Hemorrhage (17%), BUN increased (16%), Infection (16%), Alopecia (15%), Edema (13%), Elev bilirubin (13%), 1-10%, Paresthesia (2-10%), Creatinine increased (2-8%), Inj site reactions (4%), Bronchospasm (2%)
Postmarketing Reports
Cardiovascular: CHF, MI, arrhythmias, supraventricular arrhythmias
Vascular disorders: Peripheral vasculitis, gangrene, capillary leak syndrome
Skin: Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
Hepatic: Hepatic failure, hepatic veno-occlusive disease
Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, ARDS
Pulmonary: Pulmonary eosinophilia
Blood and lymphatic system: Thrombotic microangiopathy

Gemcel 200 mg Inj
Gemcel
Composition
Gemcitabine 200mg/ 1gm
Uses
Pancreatic Cancer, Non-small Cell Lung Cancer,
Breast Cancer,
Ovarian Cancer
Side Effect
Anemia (65%), Elev LFTs (68%), Neutropenia (63%), Leukopenia (62%), Pain (48%), Proteinuria (45%), Fever (41%), Hematuria (35%), Rash (30%), Thrombocytopenia (24%), Dyspnea (23%), Constipation (23%), Diarrhea (19%), Flu-like syndrome (19%), Hemorrhage (17%), BUN increased (16%), Infection (16%), Alopecia (15%), Edema (13%), Elev bilirubin (13%), 1-10%, Paresthesia (2-10%), Creatinine increased (2-8%), Inj site reactions (4%), Bronchospasm (2%)
Postmarketing Reports
Cardiovascular: CHF, MI, arrhythmias, supraventricular arrhythmias
Vascular disorders: Peripheral vasculitis, gangrene, capillary leak syndrome
Skin: Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
Hepatic: Hepatic failure, hepatic veno-occlusive disease
Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, ARDS
Pulmonary: Pulmonary eosinophilia
Blood and lymphatic system: Thrombotic microangiopathy

Oxamark 50 mg Inj
Oxamark
Composition
Oxaliplatin 50/100mg
Uses
For management of Colorectal Cancers
Side Effect
Peripheral neuropathy (76%), Anemia (64%), Nausea (64%), Fatigue (61%), Diarrhea (46%), Vomiting (37%), Abdominal pain (31%), Constipation(31%), Thrombocytopenia (30%), Fever (25%), Anorexia (20%), Leukopenia (13%), Dyspnea (13%), Cough (11%), 1-10%, Edema (10%), Neutropenia (7%), Pharyngolaryngeal dysesthesia (1-2%), <1%, Pulmonary fibrosis, Posterior leukoencephalopathy syndrome.
pathy

Oxamark 100 mg Inj
Oxamark
Composition
Oxaliplatin 50/100mg
Uses
For management of Colorectal Cancers
Side Effect
Peripheral neuropathy (76%), Anemia (64%), Nausea (64%), Fatigue (61%), Diarrhea (46%), Vomiting (37%), Abdominal pain (31%), Constipation(31%), Thrombocytopenia (30%), Fever (25%), Anorexia (20%), Leukopenia (13%), Dyspnea (13%), Cough (11%), 1-10%, Edema (10%), Neutropenia (7%), Pharyngolaryngeal dysesthesia (1-2%), <1%, Pulmonary fibrosis, Posterior leukoencephalopathy syndrome.
pathy

Paclimark 30 mg Inj
Paclimark
Composition
Paclitaxel 30/100/260mg
Novapressin contains
Uses
Breast cancer, Ovarian cancer, Non-Small cell Lung cancer, AIDS related Kaposi’s Sarcoma
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
Neutropenia (78-100%), Alopecia (55-96%), Anemia (47-96%), Arthralgia/myalgia (93%), Diarrhea (90%), Leukopenia (90%), Nausea/vomiting (9-88%), Opportunistic infections (76%), Peripheral neuropathy (42-79%), Thrombocytopenia (4-68%), Mucositis (5-45%), Hypersensitivity (2-45%), Renal impairment (34%), Hypotension (17%), 1-10%, Bradycardia (3%), <1%, Grand mal seizures, Cardiac conduction abnormalities.
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Paclimark 100 mg Inj
Paclimark
Composition
Paclitaxel 30/100/260mg
Novapressin contains
Uses
Breast cancer, Ovarian cancer, Non-Small cell Lung cancer, AIDS related Kaposi’s Sarcoma
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
Neutropenia (78-100%), Alopecia (55-96%), Anemia (47-96%), Arthralgia/myalgia (93%), Diarrhea (90%), Leukopenia (90%), Nausea/vomiting (9-88%), Opportunistic infections (76%), Peripheral neuropathy (42-79%), Thrombocytopenia (4-68%), Mucositis (5-45%), Hypersensitivity (2-45%), Renal impairment (34%), Hypotension (17%), 1-10%, Bradycardia (3%), <1%, Grand mal seizures, Cardiac conduction abnormalities.
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Paclimark 260 mg Inj
Paclimark
Composition
Paclitaxel 30/100/260mg
Novapressin contains
Uses
Breast cancer, Ovarian cancer, Non-Small cell Lung cancer, AIDS related Kaposi’s Sarcoma
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
Neutropenia (78-100%), Alopecia (55-96%), Anemia (47-96%), Arthralgia/myalgia (93%), Diarrhea (90%), Leukopenia (90%), Nausea/vomiting (9-88%), Opportunistic infections (76%), Peripheral neuropathy (42-79%), Thrombocytopenia (4-68%), Mucositis (5-45%), Hypersensitivity (2-45%), Renal impairment (34%), Hypotension (17%), 1-10%, Bradycardia (3%), <1%, Grand mal seizures, Cardiac conduction abnormalities.
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Donataxel 20 mg Inj
Donataxel
Composition
Docetaxel 20/80mg
Uses
Breast Cancer, Prostate cancer, Non-Small cell Lung cancer
Side Effect
>50% Alopecia, Anemia, Leukopenia, Neutropenia, Asthenia, 10-50%, Fever, Infections, Fluid retention, Hypersensitivity, Skin reactions, Diarrhea, Nausea, Vomiting, Sensory neuropathy, Myalgia, Nail changes, 1-10%, Arthralgia, Thrombocytopenia.

Donataxel 80 mg Inj
Donataxel
Composition
Docetaxel 20/80mg
Uses
Breast Cancer, Prostate cancer, Non-Small cell Lung cancer
Side Effect
>50% Alopecia, Anemia, Leukopenia, Neutropenia, Asthenia, 10-50%, Fever, Infections, Fluid retention, Hypersensitivity, Skin reactions, Diarrhea, Nausea, Vomiting, Sensory neuropathy, Myalgia, Nail changes, 1-10%, Arthralgia, Thrombocytopenia.

Doxite 50 mcg Inj
Doxtie
Composition
Doxorubicin 50mg
Uses
Doxorubicin is commonly used to treat some leukemias and Hodgkin’s lymphoma, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma.
Side Effect
Cough or hoarseness accompanied by fever or chills
darkening or redness of the skin (if you recently had radiation treatment)
fast or irregular heartbeat
fever or chills
joint pain
lower back or side pain accompanied by fever or chills
pain at the injection site
painful or difficult urination accompanied by fever or chills
red streaks along the injected vein
shortness of breath
stomach pain
swelling of the feet and lower legs
Rare
Black, tarry stools
blood in the urine
pinpoint red spots on the skin
unusual bleeding or bruising.

Gembio 200 mg Inj
Gembio
Composition
Gemcitabine 200mg/ 1gm
Uses
Pancreatic Cancer, Non-small Cell Lung Cancer,
Breast Cancer,
Ovarian Cancer
Side Effect
Anemia (65%), Elev LFTs (68%), Neutropenia (63%), Leukopenia (62%), Pain (48%), Proteinuria (45%), Fever (41%), Hematuria (35%), Rash (30%), Thrombocytopenia (24%), Dyspnea (23%), Constipation (23%), Diarrhea (19%), Flu-like syndrome (19%), Hemorrhage (17%), BUN increased (16%), Infection (16%), Alopecia (15%), Edema (13%), Elev bilirubin (13%), 1-10%, Paresthesia (2-10%), Creatinine increased (2-8%), Inj site reactions (4%), Bronchospasm (2%)
Postmarketing Reports
Cardiovascular: CHF, MI, arrhythmias, supraventricular arrhythmias
Vascular disorders: Peripheral vasculitis, gangrene, capillary leak syndrome
Skin: Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
Hepatic: Hepatic failure, hepatic veno-occlusive disease
Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, ARDS
Pulmonary: Pulmonary eosinophilia
Blood and lymphatic system: Thrombotic microangiopat

Gembio 1 gm Inj
Gembio
Composition
Gemcitabine 200mg/ 1gm
Uses
Pancreatic Cancer, Non-small Cell Lung Cancer,
Breast Cancer,
Ovarian Cancer
Side Effect
Anemia (65%), Elev LFTs (68%), Neutropenia (63%), Leukopenia (62%), Pain (48%), Proteinuria (45%), Fever (41%), Hematuria (35%), Rash (30%), Thrombocytopenia (24%), Dyspnea (23%), Constipation (23%), Diarrhea (19%), Flu-like syndrome (19%), Hemorrhage (17%), BUN increased (16%), Infection (16%), Alopecia (15%), Edema (13%), Elev bilirubin (13%), 1-10%, Paresthesia (2-10%), Creatinine increased (2-8%), Inj site reactions (4%), Bronchospasm (2%)
Postmarketing Reports
Cardiovascular: CHF, MI, arrhythmias, supraventricular arrhythmias
Vascular disorders: Peripheral vasculitis, gangrene, capillary leak syndrome
Skin: Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
Hepatic: Hepatic failure, hepatic veno-occlusive disease
Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, ARDS
Pulmonary: Pulmonary eosinophilia
Blood and lymphatic system: Thrombotic microangiopat

Oncofusion IV Infusion Set
Oncofusion
Uses
PVC free IV set for administration of drugs which can react with PVC in normal IV sets (eg: Paclitaxel, Docetaxel etc)

Tzmide 20 mg Inj
TZmide Capsule
Composition
Temozopamide 20/100mg. Capsule
Uses
Astrocytoma, Glioblastoma Multiforme
Side Effect
Alopecia (55-69%), Lymphopenia (55%), Nausea (53%), Vomiting (42%), Headache (41%), Fatigue (34%), Constipation (33%), Anorexia (9-27%), Convulsions (23%), Thrombocytopenia (19%), Rash (8-19%), Hemiparesis (18%), Diarrhea (16%), Neutropenia (14%), Fever (13%), Asthenia (13%), Dizziness (12%), Peripheral edema (11%), Viral infections (11%), 1-10% (selected), Amnesia (10%), Insomnia (10%), Abdominal pain (5-9%), Ataxia (8%), Back pain (8%), Paresis (8%), URI (8%), Urinary incontinence (8%), UTI (8%), Abnormal vision (5-8%), Pruritus (5-8%), Breast pain (6%), Depression (6%), Confusion (5%), Myalgia (5%), Weight gain (5%), Anemia (4%), Erythema (1%).

Tzmide 100 mg Inj
TZmide Capsule
Composition
Temozopamide 20/100mg. Capsule
Uses
Astrocytoma, Glioblastoma Multiforme
Side Effect
Alopecia (55-69%), Lymphopenia (55%), Nausea (53%), Vomiting (42%), Headache (41%), Fatigue (34%), Constipation (33%), Anorexia (9-27%), Convulsions (23%), Thrombocytopenia (19%), Rash (8-19%), Hemiparesis (18%), Diarrhea (16%), Neutropenia (14%), Fever (13%), Asthenia (13%), Dizziness (12%), Peripheral edema (11%), Viral infections (11%), 1-10% (selected), Amnesia (10%), Insomnia (10%), Abdominal pain (5-9%), Ataxia (8%), Back pain (8%), Paresis (8%), URI (8%), Urinary incontinence (8%), UTI (8%), Abnormal vision (5-8%), Pruritus (5-8%), Breast pain (6%), Depression (6%), Confusion (5%), Myalgia (5%), Weight gain (5%), Anemia (4%), Erythema (1%).

Bortemark 3.5 mg Inj
Bortemark
Composition
Bortezomib 3.5mg
Uses
Mantle cell Lymphoma, Multiple myeloma
Side Effect
Asthenia (61-65%), Nausea (61-65%), Diarrhea (51-55%), Anorexia (41-45%), Constipation (41-45%), Thrombocytopenia (41-45%), Peripheral neuropathy (IV: 16-41%; SC: 6-24%), Pyrexia (36-40%), Vomiting (36-40%), Anemia (31-35%), Arthralgia (26-30%), Headache (26-30%), Insomnia (26-30%), Limb pain (26-30%), Dizziness (21-25%), Dyspnea (21-25%), Edema (21-25%), Neutropenia (21-25%), Paresthesia (21-25%), Rash (21-25%), Cough (15-20%), Dehydration (15-20%), URI (15-20%), Rigors, grade 4 toxicity (10-15%), Frequency Not Defined, Hypotension, Anxiety, Pain, Pruritus, Abdominal pain, Dyspepsia, Back pain, Bone pain, Myalgia, Muscle spasms, Herpes zoster, Pneumonia, Blurred vision.

Photofusion IV Infusion Set
Photofusion
Uses
For administration of Photosensitive (light sensitive) drugs

Idelara 2.5 mg
IDelara
Composition
Letrozole 2.5mg tablets
Uses
Breast Cancer in Postmenopausal women, Hornone receptor Positive Breast Cancer
Side Effect
Diaphoresis (24%), Bone pain (22%), Hot flashes (19%), Back pain (18%), Dyspnea (18%), Nausea (17%), Night sweats (14%), Cough (13%), Fatigue (13%), 1-10%, Constipation (10%), Hypertension (8%), Chest pain (8%), Diarrhea (8%), Decr wt (7%), Edema (7%), Breast pain (7%), Bone fractures (6%), UTI (6%), Hypercalcemia (5%), Headache (4%), Weakness (4%), Vomiting (3%), Osteoporosis (2%).

Panataxel 30 mg
Panataxel
Novapressin contains
Terlipressin Acetate (triglycyl¬lysine-vasopressin) 1mg
Uses
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Panataxel 100 mg
Panataxel
Novapressin contains
Terlipressin Acetate (triglycyl¬lysine-vasopressin) 1mg
Uses
Novapressin is indicated in the treatment of bleeding oesophageal varices and emergency treatment of type 1 hepatorenal syndrome,as defined by IAC (International Ascites Club) criteria.
Side Effect
paleness of face and body and a slight blood pressure increase, headache and, local necrosis, abdominal pain, nausea, diarrhoea and spontaneous evacuation. Dyspnea, hyponatraemia and hypokalaemia

Anastronat Tablets 1 mg

Cyclophos-Med 200 mg

Cyclophos-Med 500 mg

Benzimir 25mg

Benzimir 100mg

Bevaas 100mg / 4ml

Bevaas 400mg / 16ml

Sukuba Inj

Bagocap 500mg

Tamoxis


Azeflumark Nasal Spray
Azeflumark Nasal Spray
Each Metered Spray Delivers:
Azelastine Hydrochloride BP 140 mcg
Fluticasone Propionate BP 50 mcg
Composition:
Azelastine Hydrochloride BP 0.14% w/v
Fluticasone Propionate BP 0.05% w/v
Preservatives: Benzalkonium Chloride Solution BP 0.02% w/v
Phenylethyl Alcohol USP 0.25% w/v
Excipients q.s
Uses
Azeflumark nasal spray is indicated for the management of symptoms of allergic rhinitis, once the need for an antihistamine and corticosteroid has
been established. It is recommended for the treatment of moderate to severe persistent symptoms in adults and adolescents above 12 years of age. It can be used for treating non- allergic vasomotor rhinitis in adults and adolescents, 12 years of age and older. For children aged 5-12 years, recommended for severe symptoms of allergic rhinitis.
Side Effect
The most likely side effects with the combination of azelastine hydrochloride and fluticasone propionate are headache, somnolence, pharyngitis, epistaxis, nasal burning/ irritation, nausea, vomiting, cough, and taste disturbances. The combination may produce a bitter taste which may lead to occasional nausea. This hitter taste usually disappears after sometime.

Metomark Nasal Spray
Metomark Nasal Spray
Each Metered Spray Delivers:
Mometasone Furoate Monohydrate
Equivalent to Mometasone Furoate USP 50 mcg
Composition:
Mometasone Furoate Monohydrate
Equivalent to Mometasone Furoate USP 0.05% w/v
Preservative
Benzalkonium Chloride Solution BP 0.02% w/v
Phenyl Ethyl Alcohol USP 0.25% w/v
Uses
Mometasone Furoate Nasal Spray is indicated for use in adults and children 12 years of age and older to treat the symptoms of seasonal allergic or perennial rhinitis.
Mometasone Furoate Nasal Spray is indicated for the treatment of nasal polyps in adults 18 years of age and older.
Mometasone Furoate Nasal Spray is also indicated or use in children 6 to 11 years of age to treat the symptoms of seasonal allergic or perennial allergic rhinitis.
In patients who have a history of moderate to severe symptoms of seasonal allergic rhinitis, prophylactic treatment with Mometasone Furoate Nasal Spray may be initiated up to four weeks prior to the anticipated start of the pollen season.
Side Effect
Treatment-related adverse events reported in clinical studies for allergic rhinitis in adult and adolescent patients are shown below (Table 1).
| Table I Allergic Rhinitis-Treatment Related Undesirable Effects for Mometasone Furoate Nasal Spray
Very common (>1/10), common (> 1 /100, < 1/10); uncommon (>1/1000, < 1/100); rare (> I/10,000, < 1/1000); very rare (<1/10,000)
| |
| Respiratory, thoracic and mediastinal disorders
Common: | Epistaxis, Pharyngitis, nasal burning, nasal irritation, nasal ulceration |
| General disorders and administration site conditions
Common: | Headache |
Epistaxis was generally self-limiting and mild in severity and occurred at a higher incidence compared to placebo (5%), but at a comparable or lower incidence when compared to the active control nasal corticosteroids studied (up to 15%). The incidence of all other effects was comparable with that of placebo.
In the pediatric population, the incidence of adverse events, e.g., epistaxis (6%), headache (3%), nasal irritation (2%) and sneezing (2%) was comparable to placebo.
In patients treated for nasal polyposis, the overall incidence of adverse events was comparable to placebo and similar to that observed for patients with allergic rhinitis.
Treatment-related adverse events reported in ≥1% of patients in clinical studies for polyposis are shown below (Table 2)
| Table 2: Polyposis-Treatment Related Undesirable Effects = 1% for Mometasone Furoate Nasal Spray
Very common (>1/10), common (> 1 /100, < 1/10); uncommon (>1/1000, < 1/100); rare (> 1/10,000, < 1/1000); very rare (<1/10,000)
| ||
| 200 mcg once a day | 200 mcg twice a day | |
| Respiratory, thoracic and mediastinal disorders
Upper respiratory tract infection Epistaxis
|
Common Common |
Uncommon Very common |
| Gastrointestinal disorders
Throat irritation |
—– |
Common |
| General disorders and administration site conditions
Headache |
Common |
Common |
In patients treated for acute rhinosinusitis, the incidence of epistaxis for Mometasone Furoate was 3.3% vs. 2.6% for placebo and similar to that observed for patients treated with allergic rhinitis.
Rarely. immediate hypersensitivity reactions, including bronchospasm and dyspnea may occur after intranasal administration of Mometasone Furoate Monohydrate. Very rarely, anaphylaxis and angioedema have been reported.
Disturbances of taste and smell have been reported very rarely.
As with other intranasal corticosteroids rare cases of nasal septum perforation have been reported.
Systemic effects of nasal corticosteroids may occur, particularly when prescribed at high doses for prolonged periods.
Rare cases of glaucoma, increased intraocular pressure and/or cataracts have been reported with the use of intranasal corticosteroids

Ltriz ODS
Ltriz ODS
Each orally disintegrating strip contains:
Levocetirizine Dihydrochloride 5 mg
Excipients q.s.
Uses
Levocetirizine is indicated for:
– the relief of nasal and ocular symptoms of seasonal and perennial allergic rhinitis;
– the relief of symptoms of chronic idiopathic urticaria.
Side Effect
Somnolence, Dizziness, Headache, Pharyngitis, Rhinitis, Abdominal pain, Dry mouth, nausea, Fatigue. Uncommon: Agitation, Paraesthesia, Diarrhoea, Pruritus, Rash, Asthenia, Malaise.
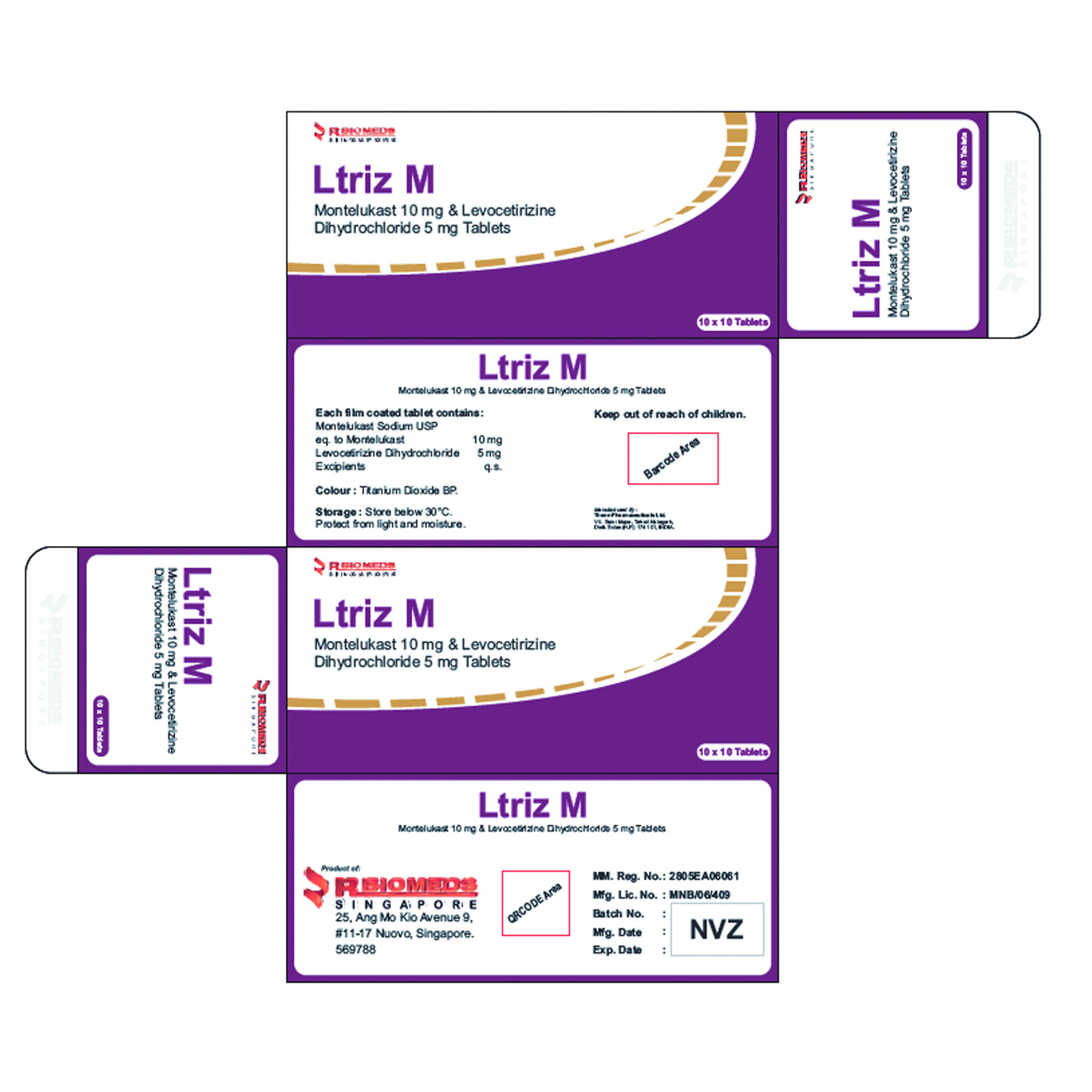
Ltriz M tablet

Azeflumark Nasal Spray
Azeflumark Nasal Spray
Each Metered Spray Delivers:
Azelastine Hydrochloride BP 140 mcg
Fluticasone Propionate BP 50 mcg
Composition:
Azelastine Hydrochloride BP 0.14% w/v
Fluticasone Propionate BP 0.05% w/v
Preservatives: Benzalkonium Chloride Solution BP 0.02% w/v
Phenylethyl Alcohol USP 0.25% w/v
Excipients q.s
Uses
Azeflumark nasal spray is indicated for the management of symptoms of allergic rhinitis, once the need for an antihistamine and corticosteroid has
been established. It is recommended for the treatment of moderate to severe persistent symptoms in adults and adolescents above 12 years of age. It can be used for treating non- allergic vasomotor rhinitis in adults and adolescents, 12 years of age and older. For children aged 5-12 years, recommended for severe symptoms of allergic rhinitis.
Side Effect
The most likely side effects with the combination of azelastine hydrochloride and fluticasone propionate are headache, somnolence, pharyngitis, epistaxis, nasal burning/ irritation, nausea, vomiting, cough, and taste disturbances. The combination may produce a bitter taste which may lead to occasional nausea. This hitter taste usually disappears after sometime.

Metomark Nasal Spray
Metomark Nasal Spray
Each Metered Spray Delivers:
Mometasone Furoate Monohydrate
Equivalent to Mometasone Furoate USP 50 mcg
Composition:
Mometasone Furoate Monohydrate
Equivalent to Mometasone Furoate USP 0.05% w/v
Preservative
Benzalkonium Chloride Solution BP 0.02% w/v
Phenyl Ethyl Alcohol USP 0.25% w/v
Uses
Mometasone Furoate Nasal Spray is indicated for use in adults and children 12 years of age and older to treat the symptoms of seasonal allergic or perennial rhinitis.
Mometasone Furoate Nasal Spray is indicated for the treatment of nasal polyps in adults 18 years of age and older.
Mometasone Furoate Nasal Spray is also indicated or use in children 6 to 11 years of age to treat the symptoms of seasonal allergic or perennial allergic rhinitis.
In patients who have a history of moderate to severe symptoms of seasonal allergic rhinitis, prophylactic treatment with Mometasone Furoate Nasal Spray may be initiated up to four weeks prior to the anticipated start of the pollen season.
Side Effect
Treatment-related adverse events reported in clinical studies for allergic rhinitis in adult and adolescent patients are shown below (Table 1).
| Table I Allergic Rhinitis-Treatment Related Undesirable Effects for Mometasone Furoate Nasal Spray
Very common (>1/10), common (> 1 /100, < 1/10); uncommon (>1/1000, < 1/100); rare (> I/10,000, < 1/1000); very rare (<1/10,000)
| |
| Respiratory, thoracic and mediastinal disorders
Common: | Epistaxis, Pharyngitis, nasal burning, nasal irritation, nasal ulceration |
| General disorders and administration site conditions
Common: | Headache |
Epistaxis was generally self-limiting and mild in severity and occurred at a higher incidence compared to placebo (5%), but at a comparable or lower incidence when compared to the active control nasal corticosteroids studied (up to 15%). The incidence of all other effects was comparable with that of placebo.
In the pediatric population, the incidence of adverse events, e.g., epistaxis (6%), headache (3%), nasal irritation (2%) and sneezing (2%) was comparable to placebo.
In patients treated for nasal polyposis, the overall incidence of adverse events was comparable to placebo and similar to that observed for patients with allergic rhinitis.
Treatment-related adverse events reported in ≥1% of patients in clinical studies for polyposis are shown below (Table 2)
| Table 2: Polyposis-Treatment Related Undesirable Effects = 1% for Mometasone Furoate Nasal Spray
Very common (>1/10), common (> 1 /100, < 1/10); uncommon (>1/1000, < 1/100); rare (> 1/10,000, < 1/1000); very rare (<1/10,000)
| ||
| 200 mcg once a day | 200 mcg twice a day | |
| Respiratory, thoracic and mediastinal disorders
Upper respiratory tract infection Epistaxis
|
Common Common |
Uncommon Very common |
| Gastrointestinal disorders
Throat irritation |
—– |
Common |
| General disorders and administration site conditions
Headache |
Common |
Common |
In patients treated for acute rhinosinusitis, the incidence of epistaxis for Mometasone Furoate was 3.3% vs. 2.6% for placebo and similar to that observed for patients treated with allergic rhinitis.
Rarely. immediate hypersensitivity reactions, including bronchospasm and dyspnea may occur after intranasal administration of Mometasone Furoate Monohydrate. Very rarely, anaphylaxis and angioedema have been reported.
Disturbances of taste and smell have been reported very rarely.
As with other intranasal corticosteroids rare cases of nasal septum perforation have been reported.
Systemic effects of nasal corticosteroids may occur, particularly when prescribed at high doses for prolonged periods.
Rare cases of glaucoma, increased intraocular pressure and/or cataracts have been reported with the use of intranasal corticosteroids

Ltriz ODS
Ltriz ODS
Each orally disintegrating strip contains:
Levocetirizine Dihydrochloride 5 mg
Excipients q.s.
Uses
Levocetirizine is indicated for:
– the relief of nasal and ocular symptoms of seasonal and perennial allergic rhinitis;
– the relief of symptoms of chronic idiopathic urticaria.
Side Effect
Somnolence, Dizziness, Headache, Pharyngitis, Rhinitis, Abdominal pain, Dry mouth, nausea, Fatigue. Uncommon: Agitation, Paraesthesia, Diarrhoea, Pruritus, Rash, Asthenia, Malaise.

Ltriz M tablet


Derise 25 mcg Inj
Derise
Composition
Darbepoetin alfa is available in 25, 40 mcg dose strengths. 25mcg/0.42 ml, 40mcg/0.40ml
Detailed composition of each strength is as under:
| S. No | Ingredient | 25mcg/0.42 ml
| 40mcg/0.40ml |
| 1 | Darbepoetin alfa (r-DNA origin) | 25 mcg | 40 mcg |
| 2 | Sodium Phosphate Monobasic Monohydrate USP | 0.89 mg | 0.85 mg |
| 3 | odium Phosphate Dibasic Anhydrous USP | 0.28 mg | 0.26 mg |
| 4 | Sodium Chloride USP | 3.44 mg | 3.27 mg |
| 5 | Polysorbate 80 USP | 0.021 mg | 0.020 mg |
| 6 | Water for Injection USP/EP | q.s. to 0.42 mL | q.s. to 0.40 mL |
Uses
Darbepoetin alfa injection is indicated for the treatment of
- Anemia with Chronic Renal Failure including patients on dialysis and patients not on dialysis
- Treatment of symptomatic anemia in adult cancer patients with non-myeloid malignancies receiving chemotherapy.
Side Effect
Chronic Renal Failure Patients
Adult Patients
Adverse reactions occurring in patients treated with Darbepoetin alfa are: Hypertension, dyspnea, peripheral edema, cough, procedural hypotension, angina pectoris, vascular access complications, Fluid overload, rash/erythema and arteriovenous graft thrombosis
Pediatric Patients
The most frequently reported serious adverse reactions with Darbepoetin alfa in clinical trials were hypertension and convulsions. The most commonly reported adverse reactions were hypertension, injection site pain, rash, and convulsions. Studies have not evaluated the effects of Darbepoetin alfa when administered to pediatric patients as the initial treatment for the anemia associated with CKD.
Cancer Patients Receiving Chemotherapy
The adverse reactions from controlled clinical studies and post-marketing experience are hypersensitivity, convulsions, hypertension, thromboembolic events, including pulmonary embolism, myocardial infarction, cerebrovascular disorders encompasses CNS hemorrhages and cerebrovascular accidents (ischemic and hemorrhagic), rash/erythema, Oedema and injection site pain.

Derise 40 mcg Inj
Derise
Composition
Darbepoetin alfa is available in 25, 40 mcg dose strengths. 25mcg/0.42 ml, 40mcg/0.40ml
Detailed composition of each strength is as under:
| S. No | Ingredient | 25mcg/0.42 ml
| 40mcg/0.40ml |
| 1 | Darbepoetin alfa (r-DNA origin) | 25 mcg | 40 mcg |
| 2 | Sodium Phosphate Monobasic Monohydrate USP | 0.89 mg | 0.85 mg |
| 3 | odium Phosphate Dibasic Anhydrous USP | 0.28 mg | 0.26 mg |
| 4 | Sodium Chloride USP | 3.44 mg | 3.27 mg |
| 5 | Polysorbate 80 USP | 0.021 mg | 0.020 mg |
| 6 | Water for Injection USP/EP | q.s. to 0.42 mL | q.s. to 0.40 mL |
Uses
Darbepoetin alfa injection is indicated for the treatment of
- Anemia with Chronic Renal Failure including patients on dialysis and patients not on dialysis
- Treatment of symptomatic anemia in adult cancer patients with non-myeloid malignancies receiving chemotherapy.
Side Effect
Chronic Renal Failure Patients
Adult Patients
Adverse reactions occurring in patients treated with Darbepoetin alfa are: Hypertension, dyspnea, peripheral edema, cough, procedural hypotension, angina pectoris, vascular access complications, Fluid overload, rash/erythema and arteriovenous graft thrombosis
Pediatric Patients
The most frequently reported serious adverse reactions with Darbepoetin alfa in clinical trials were hypertension and convulsions. The most commonly reported adverse reactions were hypertension, injection site pain, rash, and convulsions. Studies have not evaluated the effects of Darbepoetin alfa when administered to pediatric patients as the initial treatment for the anemia associated with CKD.
Cancer Patients Receiving Chemotherapy
The adverse reactions from controlled clinical studies and post-marketing experience are hypersensitivity, convulsions, hypertension, thromboembolic events, including pulmonary embolism, myocardial infarction, cerebrovascular disorders encompasses CNS hemorrhages and cerebrovascular accidents (ischemic and hemorrhagic), rash/erythema, Oedema and injection site pain.

Eritrogen 2000 IU Inj
Eritrogen

Eritrogen 4000 IU Inj
Eritrogen

Derise 25 mcg Inj
Derise
Composition
Darbepoetin alfa is available in 25, 40 mcg dose strengths. 25mcg/0.42 ml, 40mcg/0.40ml
Detailed composition of each strength is as under:
| S. No | Ingredient | 25mcg/0.42 ml
| 40mcg/0.40ml |
| 1 | Darbepoetin alfa (r-DNA origin) | 25 mcg | 40 mcg |
| 2 | Sodium Phosphate Monobasic Monohydrate USP | 0.89 mg | 0.85 mg |
| 3 | odium Phosphate Dibasic Anhydrous USP | 0.28 mg | 0.26 mg |
| 4 | Sodium Chloride USP | 3.44 mg | 3.27 mg |
| 5 | Polysorbate 80 USP | 0.021 mg | 0.020 mg |
| 6 | Water for Injection USP/EP | q.s. to 0.42 mL | q.s. to 0.40 mL |
Uses
Darbepoetin alfa injection is indicated for the treatment of
- Anemia with Chronic Renal Failure including patients on dialysis and patients not on dialysis
- Treatment of symptomatic anemia in adult cancer patients with non-myeloid malignancies receiving chemotherapy.
Side Effect
Chronic Renal Failure Patients
Adult Patients
Adverse reactions occurring in patients treated with Darbepoetin alfa are: Hypertension, dyspnea, peripheral edema, cough, procedural hypotension, angina pectoris, vascular access complications, Fluid overload, rash/erythema and arteriovenous graft thrombosis
Pediatric Patients
The most frequently reported serious adverse reactions with Darbepoetin alfa in clinical trials were hypertension and convulsions. The most commonly reported adverse reactions were hypertension, injection site pain, rash, and convulsions. Studies have not evaluated the effects of Darbepoetin alfa when administered to pediatric patients as the initial treatment for the anemia associated with CKD.
Cancer Patients Receiving Chemotherapy
The adverse reactions from controlled clinical studies and post-marketing experience are hypersensitivity, convulsions, hypertension, thromboembolic events, including pulmonary embolism, myocardial infarction, cerebrovascular disorders encompasses CNS hemorrhages and cerebrovascular accidents (ischemic and hemorrhagic), rash/erythema, Oedema and injection site pain.

Derise 40 mcg Inj
Derise
Composition
Darbepoetin alfa is available in 25, 40 mcg dose strengths. 25mcg/0.42 ml, 40mcg/0.40ml
Detailed composition of each strength is as under:
| S. No | Ingredient | 25mcg/0.42 ml
| 40mcg/0.40ml |
| 1 | Darbepoetin alfa (r-DNA origin) | 25 mcg | 40 mcg |
| 2 | Sodium Phosphate Monobasic Monohydrate USP | 0.89 mg | 0.85 mg |
| 3 | odium Phosphate Dibasic Anhydrous USP | 0.28 mg | 0.26 mg |
| 4 | Sodium Chloride USP | 3.44 mg | 3.27 mg |
| 5 | Polysorbate 80 USP | 0.021 mg | 0.020 mg |
| 6 | Water for Injection USP/EP | q.s. to 0.42 mL | q.s. to 0.40 mL |
Uses
Darbepoetin alfa injection is indicated for the treatment of
- Anemia with Chronic Renal Failure including patients on dialysis and patients not on dialysis
- Treatment of symptomatic anemia in adult cancer patients with non-myeloid malignancies receiving chemotherapy.
Side Effect
Chronic Renal Failure Patients
Adult Patients
Adverse reactions occurring in patients treated with Darbepoetin alfa are: Hypertension, dyspnea, peripheral edema, cough, procedural hypotension, angina pectoris, vascular access complications, Fluid overload, rash/erythema and arteriovenous graft thrombosis
Pediatric Patients
The most frequently reported serious adverse reactions with Darbepoetin alfa in clinical trials were hypertension and convulsions. The most commonly reported adverse reactions were hypertension, injection site pain, rash, and convulsions. Studies have not evaluated the effects of Darbepoetin alfa when administered to pediatric patients as the initial treatment for the anemia associated with CKD.
Cancer Patients Receiving Chemotherapy
The adverse reactions from controlled clinical studies and post-marketing experience are hypersensitivity, convulsions, hypertension, thromboembolic events, including pulmonary embolism, myocardial infarction, cerebrovascular disorders encompasses CNS hemorrhages and cerebrovascular accidents (ischemic and hemorrhagic), rash/erythema, Oedema and injection site pain.

Eritrogen 2000 IU Inj
Eritrogen

Eritrogen 4000 IU Inj
Eritrogen

Tocilizumab

Tocilizumab


Fluritop Mouthwash
Fluoritop Mouthwash
Composition
200ppm (0.04%) of Sodium fluoride
Uses
Anticavity fluoride mouthwash

Hexidine Mouthwash
Hexidine (80ml & 160ml)
Composition
Chlorhexidine gluconate 0.2%
Uses
Gold standard Antibacterial antiplaque mouthwash
Prevents dental infections, plaque formation, helps to reduce risk of Covid 19 infection
Alcohol free

RA Thermoseal Gel
RA Thermoseal Gel (15&100gm)
Composition
Potassium Nitrate 5% Sodium monoflurophosphate 0.7%
Indications
For dentinal Hypersensitivity
Gel formula has advantage of less foaming which prevents friction while brushing teeth.
RA Thermoseal Paste
RA Thermoseal paste (15gm, 50gm & 100gm)
Composition
Potassium nitrate 5%, Sodium monoflurophosphate 0.7%
Uses
For Rapid relief from sensitivity
95% patients experience no/slight pain starting from Day 1 of use

Thermoseal Repair
Thermoseal Repair Paste (15&100gm)
Composition
Strontium chloride hexahydrate 10%
Uses
For repair and maintenance of teeth
Prevents wear and tear of teeth

Kids Bunny Gel
Kids bunny dental gel(15gm & 80gm)
Composition
Sodium monoflurophosphate 0.3%
Uses
Anticavity toothpaste for Kids
Available in delicious strawberry mint flavor which encourages children to brush teeth

Kids Dyny Brush
Kids Dyny Brush
Tooth brush for children
Uses
Small amd soft bristles which ensure complete cleaning of oral cavity of children without any injuries
Attractive dinosaur handle helps children to like this brush

Thermoseal Tooth Brush
Thermoseal Tooth Brush
Uses
Toothbrush for regular use which ensures complete cleaning of Oral cavity

Thermoseal Smart Tooth Brush
Thermoseal smart brush
Uses
Toothbrush with tongue cleaner at back
Contoured rubber grip makes it easier to handle and use
Rounded head ensures complete cleaning of oral cavity
Thermoseal Ultrasoft
Thermoseal Ultrasoft
Uses
Has very soft bristles which makes it a superior option for patients having dentinal Hypersensitivity, bleeding gums etc

Thermoseal Ortho Brush
Thermoseal Ortho Brush
Uses
Professionally designed brush with long outer bristles and short inner bristles which ensure complete cleaning of and around Orthodontic implants

Clinsodent Brush
Clinsodent toothbrush
Uses
For cleaning of dentures in old people
Thermoseal Proxa NS
Thermoseal Proxa NS
Uses
For effective cleaning of Inter-dental spaces
Younifloss
Younifloss
Uses
Floss for cleaning interdental spaces
Handy and convenient to use and carry
Thermoseal Floss
Thermoseal Floss 50 mtr
50 mtr floss for effective cleaning of Inter-dental spaces
Uses
Floss has unique advantage of thickening on coming in contact with Saliva
Mint flavoured

Wassup ( Mint )
Wassup spray
Uses
Mint flavoured oral spray to fight bad breath

Shine N Smile
Shine N smile (15gm & 80gm)
Uses
Tooth polish for whitening the teeth

Orofresh Toothpaste (For Extra Freshness)
Orofresh extra fresh toothpaste
Composition
Sodium monoflurophosphate 0.7%
Uses
Helps in preventing dental caries, decay and refreshing mint flavor helps to fight bad breath

Orofresh Toothpaste (For Sensitivity)
Orofresh Sensitive Toothpaste (20gm & 100gm)
Composition
Potassium nitrate 5%, Sodium monoflurophosphate 0.7%
Uses
For Rapid relief from sensitivity
95% patients experience no/slight pain starting from Day 1 of use
Orofresh Toothpaste (For Kids)
Orofresh Toothpaste (For Kids)
Composition
Sodium monoflurophosphate 0.3%
Uses
Helps to fight cavities, caries and decay in children aged 2yrs and above
Delicious Strawberry flavour encourages children to like the taste and aroma of toothpaste as well as helps fight bad breath

Orofresh Mouthwash
Orofresh Mouthwash(100ml & 200ml)
Composition
Chlorhexidine gluconate 0.2%, Zinc 5%, Sodium Fluoride
Uses
Antibacterial, anticavity, antiplaque mouthwash with Zinc Prevents dental infections, plaque formation, helps to reduce risk of Covid 19 infection
Refreshing green tea flavour

Orofresh Herbal Toothpaste (20gm, 50gm, 100gm)

Orofresh Mouth Freshner

Fluritop Mouthwash
Fluoritop Mouthwash
Composition
200ppm (0.04%) of Sodium fluoride
Uses
Anticavity fluoride mouthwash

Hexidine Mouthwash
Hexidine (80ml & 160ml)
Composition
Chlorhexidine gluconate 0.2%
Uses
Gold standard Antibacterial antiplaque mouthwash
Prevents dental infections, plaque formation, helps to reduce risk of Covid 19 infection
Alcohol free

RA Thermoseal Gel
RA Thermoseal Gel (15&100gm)
Composition
Potassium Nitrate 5% Sodium monoflurophosphate 0.7%
Indications
For dentinal Hypersensitivity
Gel formula has advantage of less foaming which prevents friction while brushing teeth.

RA Thermoseal Paste
RA Thermoseal paste (15gm, 50gm & 100gm)
Composition
Potassium nitrate 5%, Sodium monoflurophosphate 0.7%
Uses
For Rapid relief from sensitivity
95% patients experience no/slight pain starting from Day 1 of use

Thermoseal Repair
Thermoseal Repair Paste (15&100gm)
Composition
Strontium chloride hexahydrate 10%
Uses
For repair and maintenance of teeth
Prevents wear and tear of teeth

Kids Bunny Gel
Kids bunny dental gel(15gm & 80gm)
Composition
Sodium monoflurophosphate 0.3%
Uses
Anticavity toothpaste for Kids
Available in delicious strawberry mint flavor which encourages children to brush teeth

Kids Dyny Brush
Kids Dyny Brush
Tooth brush for children
Uses
Small amd soft bristles which ensure complete cleaning of oral cavity of children without any injuries
Attractive dinosaur handle helps children to like this brush

Thermoseal Tooth Brush
Thermoseal Tooth Brush
Uses
Toothbrush for regular use which ensures complete cleaning of Oral cavity

Thermoseal Smart Tooth Brush
Thermoseal smart brush
Uses
Toothbrush with tongue cleaner at back
Contoured rubber grip makes it easier to handle and use
Rounded head ensures complete cleaning of oral cavity

Thermoseal Ultrasoft
Thermoseal Ultrasoft
Uses
Has very soft bristles which makes it a superior option for patients having dentinal Hypersensitivity, bleeding gums etc

Thermoseal Ortho Brush
Thermoseal Ortho Brush
Uses
Professionally designed brush with long outer bristles and short inner bristles which ensure complete cleaning of and around Orthodontic implants

Clinsodent Brush
Clinsodent toothbrush
Uses
For cleaning of dentures in old people

Thermoseal Proxa NS
Thermoseal Proxa NS
Uses
For effective cleaning of Inter-dental spaces

Younifloss
Younifloss
Uses
Floss for cleaning interdental spaces
Handy and convenient to use and carry

Thermoseal Floss
Thermoseal Floss 50 mtr
50 mtr floss for effective cleaning of Inter-dental spaces
Uses
Floss has unique advantage of thickening on coming in contact with Saliva
Mint flavoured

Wassup ( Mint )
Wassup spray
Uses
Mint flavoured oral spray to fight bad breath

Shine N Smile
Shine N smile (15gm & 80gm)
Uses
Tooth polish for whitening the teeth

Orofresh Toothpaste (For Extra Freshness)
Orofresh extra fresh toothpaste
Composition
Sodium monoflurophosphate 0.7%
Uses
Helps in preventing dental caries, decay and refreshing mint flavor helps to fight bad breath

Orofresh Toothpaste (For Sensitivity)
Orofresh Sensitive Toothpaste (20gm & 100gm)
Composition
Potassium nitrate 5%, Sodium monoflurophosphate 0.7%
Uses
For Rapid relief from sensitivity
95% patients experience no/slight pain starting from Day 1 of use

Orofresh Toothpaste (For Kids)
Orofresh Toothpaste (For Kids)
Composition
Sodium monoflurophosphate 0.3%
Uses
Helps to fight cavities, caries and decay in children aged 2yrs and above
Delicious Strawberry flavour encourages children to like the taste and aroma of toothpaste as well as helps fight bad breath

Orofresh Mouthwash
Orofresh Mouthwash(100ml & 200ml)
Composition
Chlorhexidine gluconate 0.2%, Zinc 5%, Sodium Fluoride
Uses
Antibacterial, anticavity, antiplaque mouthwash with Zinc Prevents dental infections, plaque formation, helps to reduce risk of Covid 19 infection
Refreshing green tea flavour

Orofresh Herbal Toothpaste (20gm, 50gm, 100gm)

Orofresh Mouth Freshner


Markvir Cream 15 gm

Clobmarksans Cream 10 gm
Clobetamark Cream 15 gm
Quench Cream
Vorif 200 mg

Virq 250 mg
Icon 100 mg
Acylex 200 mg
Acylex 400 mg

Gro-Hair (60ml & 100ml)

Markvir Cream 15 gm

Clobmarksans Cream 10 gm

Clobetamark Cream 15 gm

Quench Cream

Vorif 200 mg

Virq 250 mg

Icon 100 mg

Acylex 200 mg

Acylex 400 mg

Gro-Hair (60ml & 100ml)


Tocilizumab
Trifamox 750 mg lnj
Trifamox IBL 750
Each vial contains: amoxicillin (as sodium amoxicillin) 500mg, sulbactam (as sulbactam sodium) 250mg.
Each ampoule contains: 5ml sterile distilled water.
Uses
Trifamox IBL is specifically recommended for the treatment of infections due to microorganisms resistant to betalactamic and cephalosporinic antibiotic single therapy, due to their betalacatmase producing capacity.
It is recommended that adequate microbiological tests (cultures and susceptibility tests) be performed before treatment start in order to identify the microorganisms that cause the infection and determine their susceptibility to Trifamox IBL.
Treatment can be started before the results of bacterial tests become available when there re reasons to believe that betalactamase producing organisms are involved as etiological agents. Once the results are available, the treatment
can be modified, if necessary.
Side Effect
When administered at the recommended dosage, the drug is generally well-tolerated. Some patients may develop untoward reactions of different types and severity. The following adverse reactions have been reported:
Gastrointestinal disorders: nausea, vomiting, diarrhea, dyspepsia and epigastralgia
Hypersensitivity reactions: urticaria, Quincke’s edema, skin maculopapular rash and rarely, anaphylactic shock.
Interstitial nephritis
Hematologic reactions: neutropenia, eosinophilia, anemia and platelet dysfunction
Oral candidiasis – or other areas – as an expression of normal flora alteration
Exceptionally, Stevens-Johnson syndrome and erythema multiforme
Cases of pseudomembranous colitis have been reported with the use of aminopenicillins.
Trifamox 1500 mg lnj
Trifamox IBL 1500
Each vial contains: amoxicillin (as sodium amoxicillin) 1.000mg, sulbactam (as sulbactam sodium) 500mg.
Each ampoule contains: 5ml sterile distilled water.
Uses
Trifamox IBL is specifically recommended for the treatment of infections due to microorganisms resistant to betalactamic and cephalosporinic antibiotic single therapy, due to their betalacatmase producing capacity.
It is recommended that adequate microbiological tests (cultures and susceptibility tests) be performed before treatment start in order to identify the microorganisms that cause the infection and determine their susceptibility to Trifamox IBL.
Treatment can be started before the results of bacterial tests become available when there re reasons to believe that betalactamase producing organisms are involved as etiological agents. Once the results are available, the treatment
can be modified, if necessary.
Side Effect
When administered at the recommended dosage, the drug is generally well-tolerated. Some patients may develop untoward reactions of different types and severity. The following adverse reactions have been reported:
Gastrointestinal disorders: nausea, vomiting, diarrhea, dyspepsia and epigastralgia
Hypersensitivity reactions: urticaria, Quincke’s edema, skin maculopapular rash and rarely, anaphylactic shock.
Interstitial nephritis
Hematologic reactions: neutropenia, eosinophilia, anemia and platelet dysfunction
Oral candidiasis – or other areas – as an expression of normal flora alteration
Exceptionally, Stevens-Johnson syndrome and erythema multiforme
Cases of pseudomembranous colitis have been reported with the use of aminopenicillins.
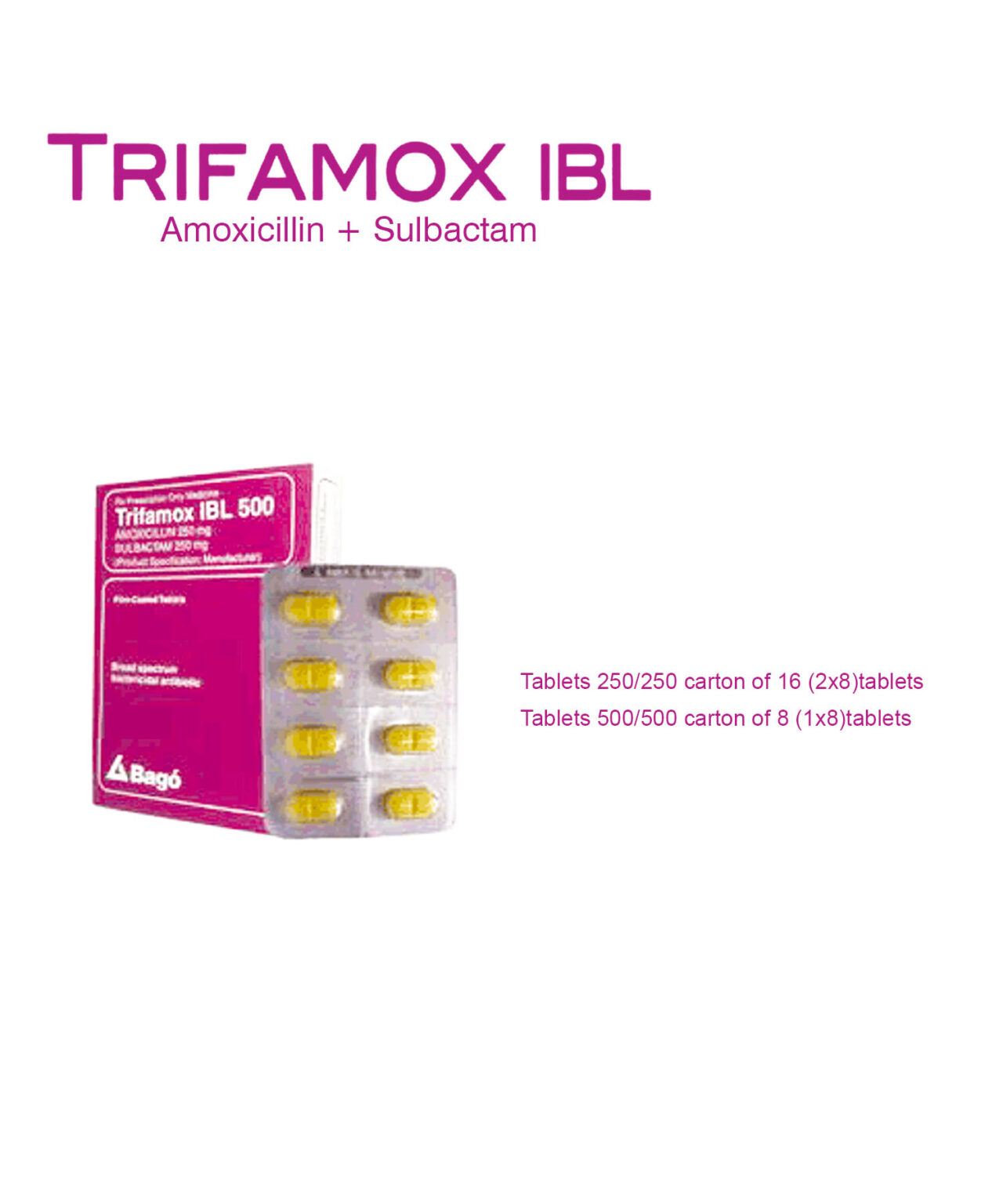
Trifamox 500 mg Tab
Trifamox IBL 500
Composition
Each Film – Coated Tablet contains:
Amoxicillin (as Amoxicillin Trihydrate) 250 mg; Sulbactam (as Pivsulbactam) 250 mg
Excipients: Croscarmellose Sodium; Magnesium Stearate; Colloidal Anhydrous Silica; Lactose, Hypromellose; Titanium Dioxide; Triacetin; Yellow 10 Ferric Oxide; Microcrystalline Cellulose
Uses
Trifamox IBL is specifically recommended for the treatment of infections due to microorganisms resistant to betalactamic and cephalosporinic antibiotic single therapy, due to their betalacatmase producing capacity.
It is recommended that adequate microbiological tests (cultures and susceptibility tests) be performed before treatment start in order to identify the microorganisms that cause the infection and determine their susceptibility to Trifamox IBL.
Treatment can be started before the results of bacterial tests become available when there re reasons to believe that betalactamase producing organisms are involved as etiological agents. Once the results are available, the treatment
can be modified, if necessary.
Side Effect
When administered at the recommended dosage, the drug is generally well-tolerated. Some patients may develop untoward reactions of different types and severity. The following adverse reactions have been reported:
Gastrointestinal disorders: nausea, vomiting, diarrhea, dyspepsia and epigastralgia
Hypersensitivity reactions: urticaria, Quincke’s edema, skin maculopapular rash and rarely, anaphylactic shock.
Interstitial nephritis
Hematologic reactions: neutropenia, eosinophilia, anemia and platelet dysfunction
Oral candidiasis – or other areas – as an expression of normal flora alteration
Exceptionally, Stevens-Johnson syndrome and erythema multiforme
Cases of pseudomembranous colitis have been reported with the use of aminopenicillins.
cillins.

Clarion 250 mg Tab
Clarion 250mg/500 mg
Composition
Each tablet contains clarithromycin 250 mg / 500 mg
Uses
H-pylori infection
Tonsillitis / Pharyngitis, AECB, Pneumonia and uncomplicated skin and soft tissue infections
Side Effect
The most frequently reported side effects include diarrhea, nausea, abnormal taste, dyspepsia, abdominal pain / discomfort and headache. The post marketing experience has attributed some allergic side effects to clarithromycin, ranging from urticaria to rare anaphylaxis

Clarion 500 mg Tab
Clarion 250mg/500 mg
Composition
Each tablet contains clarithromycin 250 mg / 500 mg
Uses
H-pylori infection
Tonsillitis / Pharyngitis, AECB, Pneumonia and uncomplicated skin and soft tissue infections
Side Effect
The most frequently reported side effects include diarrhea, nausea, abnormal taste, dyspepsia, abdominal pain / discomfort and headache. The post marketing experience has attributed some allergic side effects to clarithromycin, ranging from urticaria to rare anaphylaxis
.

Levo 250 mg Tab
Levo
Composition
Each film coated Tablet contains levofloxacin (as hemihydrate): 200mg /500 mg
Uses
Levo is indicated for the treatment of mild, moderate, and severe infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Acute bacterial sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis
Complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coil, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa
Uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus
Side Effect
The following events were considered likely to be drug-related in patients receiving levofloxacin
nausea, diarrhea, vaginitis, insomnia, abdominal pain, flatulence, pruritus , dizziness , rash, dyspepsia , genital moniliasis, moniliasis, taste perversion, vomiting , constipation, fungal infection , genital pruritis, headache , nervousness , rash erythematous , urticaria , anorexia , somnolence, agitation, rash maculo-papular, dry mouth , tremor, condition aggravated, allergic reaction.

Levo 500 mg Tab
Levo
Composition
Each film coated Tablet contains levofloxacin (as hemihydrate): 200mg /500 mg
Uses
Levo is indicated for the treatment of mild, moderate, and severe infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Acute bacterial sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis
Complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coil, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa
Uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus
Side Effect
The following events were considered likely to be drug-related in patients receiving levofloxacin
nausea, diarrhea, vaginitis, insomnia, abdominal pain, flatulence, pruritus , dizziness , rash, dyspepsia , genital moniliasis, moniliasis, taste perversion, vomiting , constipation, fungal infection , genital pruritis, headache , nervousness , rash erythematous , urticaria , anorexia , somnolence, agitation, rash maculo-papular, dry mouth , tremor, condition aggravated, allergic reaction.

Eleva Plus 1gm lnj
Eleva Plus
Composition
Eleva Plus Injection 1 gm Each vial contains:
Cefoperazone (Sodium) U.S.P. . 0.5gm
Sulbactam (Sodium) U.S.P 0.5gm
Eleva Plus Injection 2gm
Each vial contains:
Cefoperazone (Sodium) U.S.P. 1gm
Sulbactam (Sodium) U.S.P. 1gm
Uses
Mono-Therapy
Sulbactam/Cefoperazone (Eleva Plus) is indicated for the treatment of the following infections when caused by susceptible organisms: Respiratory Tract infections (Upper and Lower), Urinary Tract Infections, Peritonitis, Cholecystitis, Cholangitis, and other intra-Abdominal Infections, Septicemia, Meningitis, Skin and Soft Tissue infections, Bone and Joint Infections, Pelvic Inflammatory Disease, Endometritis, Gonorrhea, and Other Infections of the Genital Tract.
Combination Therapy
Because of the broad spectrum of activity of Sulbactam/Cefoperazone (Eleva Plus), most infections can be treated adequately with this antibiotic alone. However, Sulbactam/Cefoperazone may be used concomitantly with other antibiotics if such combinations are indicated. If an aminoglycoside is used renal function should be monitored during the course of therapy.
Side Effect
Sulbactam/Cefoperazone (Eleva Plus) is generally well-tolerated. The majority of adverse events are of mild or moderate severity are tolerated with continued treatment. The most frequent side effects observed with Sulbactam/Cefoperazone (Eleva Plus) are gastrointestinal. Others include dermatologic reactions, headache, injection pain, chills and anaphylactoid reactions.

Eleva Plus 2gm lnj
Eleva Plus
Composition
Eleva Plus Injection 1 gm Each vial contains:
Cefoperazone (Sodium) U.S.P. . 0.5gm
Sulbactam (Sodium) U.S.P 0.5gm
Eleva Plus Injection 2gm
Each vial contains:
Cefoperazone (Sodium) U.S.P. 1gm
Sulbactam (Sodium) U.S.P. 1gm
Uses
Mono-Therapy
Sulbactam/Cefoperazone (Eleva Plus) is indicated for the treatment of the following infections when caused by susceptible organisms: Respiratory Tract infections (Upper and Lower), Urinary Tract Infections, Peritonitis, Cholecystitis, Cholangitis, and other intra-Abdominal Infections, Septicemia, Meningitis, Skin and Soft Tissue infections, Bone and Joint Infections, Pelvic Inflammatory Disease, Endometritis, Gonorrhea, and Other Infections of the Genital Tract.
Combination Therapy
Because of the broad spectrum of activity of Sulbactam/Cefoperazone (Eleva Plus), most infections can be treated adequately with this antibiotic alone. However, Sulbactam/Cefoperazone may be used concomitantly with other antibiotics if such combinations are indicated. If an aminoglycoside is used renal function should be monitored during the course of therapy.
Side Effect
Sulbactam/Cefoperazone (Eleva Plus) is generally well-tolerated. The majority of adverse events are of mild or moderate severity are tolerated with continued treatment. The most frequent side effects observed with Sulbactam/Cefoperazone (Eleva Plus) are gastrointestinal. Others include dermatologic reactions, headache, injection pain, chills and anaphylactoid reactions.

Azeath 250 mg Tab
Azeath
Composition
Each film coated Tablet contains azithromycin USP
(as azithromycin dihydrate USP) 250/500mg
Excipients q.s
Uses
Azeath is indicated for the following bacterial infection induced by microorganisms susceptible to azithromycin
- Acute bacterial sinusitis (adequately diagnosed)
- Acute bacterial otitis media (adequately diagnosed) Pharyngitis, tonsillitis
- Acute exacerbation chronic bronchitis (adequately diagnosed)
- Mild to moderately severe community acquired pneumonia
- Infections of the skin and soft tissues of mild to moderate severity e.g. folliculitis, cellulitis erysipelas
- Uncomplicated Chlamydia trachomatis urethritis and cervicitis
Side Effect
Very common: Diarrhoea
Common: Changes in the numbers of some white blood cells and blood bicarbonate Headache Vomiting, stomach pain, feeling sick
Uncommon: Allergic reactions, Loss of appetite. Sight disorders, Ear disorders, Hot flush. Difficulty breathing, Chest pain, swelling, feeling unwell, weakness, tiredness
Rare: Agitation, Abnormal liver function, jaundice, Reddening and blistering of the skin when exposed to sunlight

Azeath 500 mg Tab
Azeath
Composition
Each film coated Tablet contains azithromycin USP
(as azithromycin dihydrate USP) 250/500mg
Excipients q.s
Uses
Azeath is indicated for the following bacterial infection induced by microorganisms susceptible to azithromycin
- Acute bacterial sinusitis (adequately diagnosed)
- Acute bacterial otitis media (adequately diagnosed) Pharyngitis, tonsillitis
- Acute exacerbation chronic bronchitis (adequately diagnosed)
- Mild to moderately severe community acquired pneumonia
- Infections of the skin and soft tissues of mild to moderate severity e.g. folliculitis, cellulitis erysipelas
- Uncomplicated Chlamydia trachomatis urethritis and cervicitis
Side Effect
Very common: Diarrhoea
Common: Changes in the numbers of some white blood cells and blood bicarbonate Headache Vomiting, stomach pain, feeling sick
Uncommon: Allergic reactions, Loss of appetite. Sight disorders, Ear disorders, Hot flush. Difficulty breathing, Chest pain, swelling, feeling unwell, weakness, tiredness
Rare: Agitation, Abnormal liver function, jaundice, Reddening and blistering of the skin when exposed to sunlight
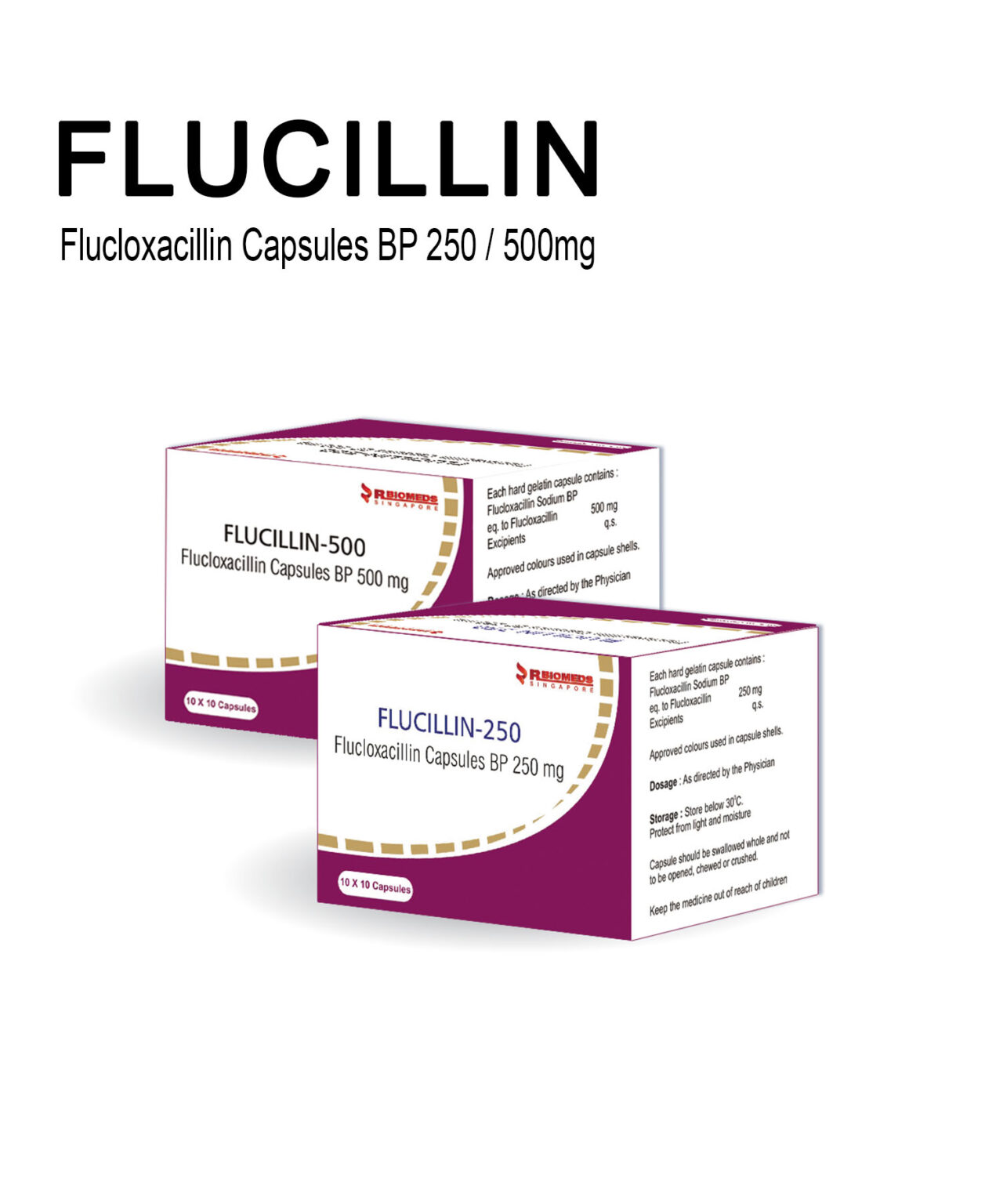
Flucillin 250 mg Cap
Flucillin
Each hard gelatin capsule contains:
Flucloxacillin Sodium BP
1.to Flucloxacillin ..250 mg/500 mg
Excipients q s.
Uses
Treatment of infections due to sensitive Gram-positive organisms. including infections caused by beta-lactamase-producing Staphylococci, Streptococci.Flucloxacillin is also indicated for use as a prophylactic during major surgical procedures such as cardiothoracic and orthopedic surgery.
Side Effect
The following convention has been utilized for the classification of undesirable effects: Blood and lymphatic system disorders, Immune system disorders, Gastrointestinal disorders, Hepato-biliary disorders, Skin and subcutaneous tissue disorders, Musculoskeletal and connective tissue disorders, Renal and urinary disorders.
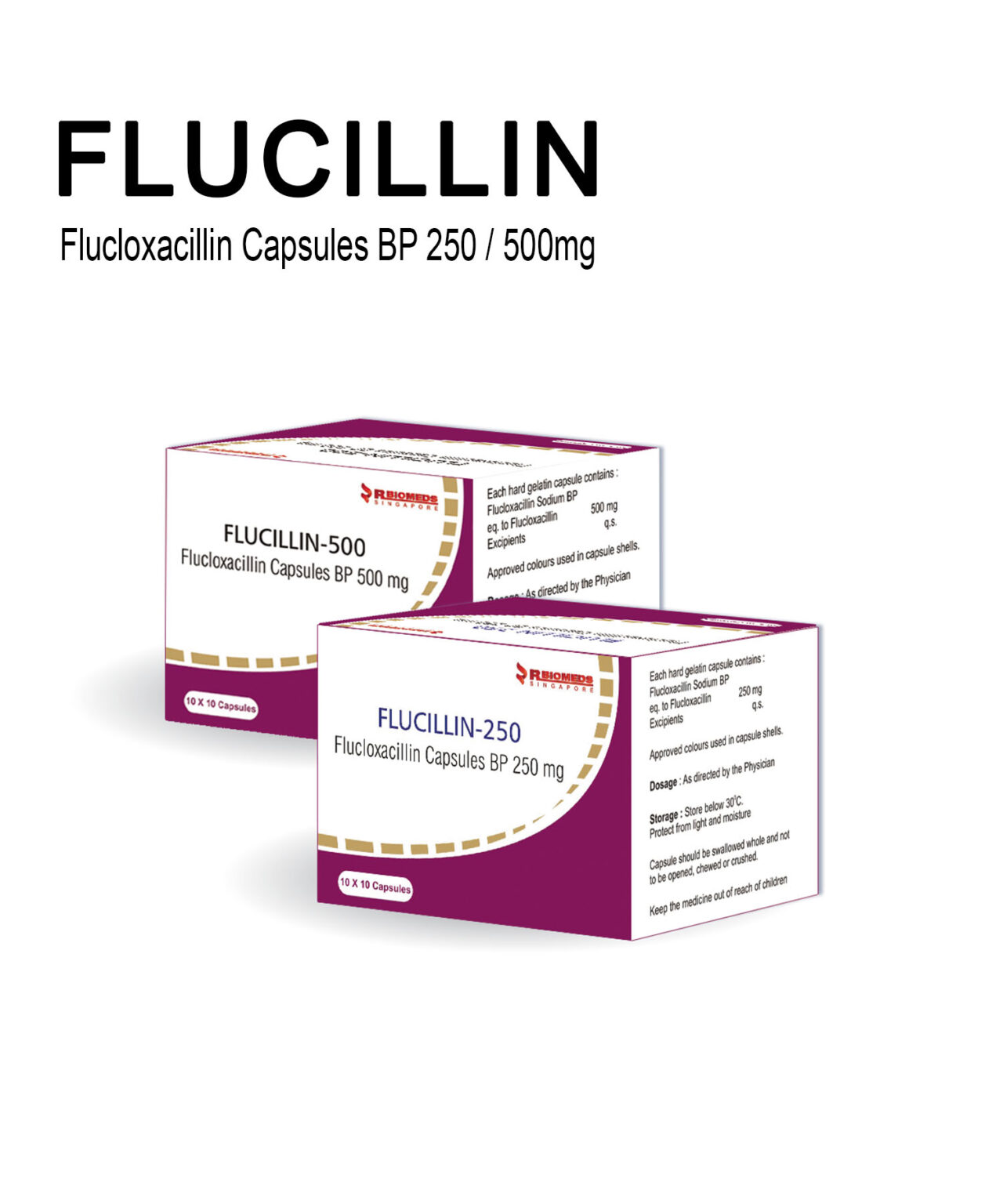
Flucillin 500 mg Cap
Flucillin
Each hard gelatin capsule contains:
Flucloxacillin Sodium BP
- to Flucloxacillin ..250 mg/500 mg
Excipients q s.
Uses
Treatment of infections due to sensitive Gram-positive organisms. including infections caused by beta-lactamase-producing Staphylococci, Streptococci.Flucloxacillin is also indicated for use as a prophylactic during major surgical procedures such as cardiothoracic and orthopedic surgery.
Side Effect
The following convention has been utilized for the classification of undesirable effects: Blood and lymphatic system disorders, Immune system disorders, Gastrointestinal disorders, Hepato-biliary disorders, Skin and subcutaneous tissue disorders, Musculoskeletal and connective tissue disorders, Renal and urinary disorders.

Theomycin 250 mg Tab
Theomycin
Each film coated tablet contains:
Erythromycin Stearate USP
- to Erythromycin 250 mg/500 mg
Excipients q.s.
Uses
Erythromycin is used for the prophylaxis and treatment of infections caused by erythromycin-sensitive organisms.
Erythromycin is highly effective in the treatment of a great variety of clinical infections such as:
Upper Respiratory Tract infections: tonsillitis, peritonsillar abscess, pharyngitis, laryngitis, sinusitis, secondary infections in influenza and common colds
Lower Respiratory Tract infections: tracheitis, acute and chronic bronchitis, pneumonia (lobar pneumonia, bronchopneumonia, primary atypical pneumonia), bronchiectasis, Legionnaire’s disease
Ear infection: otitis media and otitis externa, mastoiditis
Oral infections: gingivitis, Vincent’s angina
Eye infections: blepharitis
Skin and soft tissue infections: boils and carbuncles, paronychia, abscesses, pustular acne, impetigo, cellulitis, erysipelas
Gastrointestinal infections: cholecystitis, staphylococcal enterocolitis
Prophylaxis: pre- and post- operative trauma, burns, rheumatic fever
Other infections: osteomyelitis, urethritis, gonorrhea, syphilis, lymphogranuloma venereum, diphtheria, prostatitis, scarlet fever.
Side Effect
Blood and lymphatic system disorders:
Eosinophilia.
Cardiac disorders
QTc interval prolongation, torsades de pointes, palpitations, and cardiac rhythm disorders including ventricular tachyarrhythmias.
Ear and labyrinth disorders
Deafness, tinnitus
There have been isolated reports of reversible hearing loss occurring chiefly in patients with renal insufficiency or high doses.
Gastrointestinal disorders
The most frequent side effects of oral erythromycin preparations are gastrointestinal and are dose-related. The following have been reported:
upper abdominal discomfort, nausea, vomiting, diarrhea, pancreatitis, anorexia, infantile hypertrophic pyloric stenosis.
Pseudomembranous colitis has been rarely reported in association with erythromycin therapy.
General disorders and administration site conditions
Chest pain, fever, malaise.
Hepatobiliary disorders
Cholestatic hepatitis, jaundice, hepatic disfunction, hepatomegaly, hepatic failure, hepatocellular hepatitis.
Immune system disorders
Allergic reactions
Investigations
Increased liver enzyme values
Nervous system disorders
There have been isolated reports of transient central nervous system side effects including confusion, seizures and vertigo; however, a cause and effect relationship has not been established.
Psychiatric disorders, Hallucinations, Renal and urinary disorders, Interstitial nephritis, Skin and subcutaneous tissue disorders,
Skin eruptions, pruritus, urticaria, exanthema, angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme.
Vascular disorders, Hypotension.

Zeekan
Zeekan
Composition
ZEEKAN 200mg/5ml Suspension
Each 5ml contains:
Azithromycin (as Dihydrate) U.S.P. 200mg
Uses
Azithromycin (ZEEKAN) is active against both Gram-positive and Gram-negative organisms. It is indicated for the treatment of infections caused by susceptible bacteria in conditions listed below:
Upper Respiratory Tract Infection: Acute Pharyngitis, Acute Tonsillitis, Sinusitis and Acute Otitis media.
Lower Respiratory Tract Infections: Acute bronchitis, Acute bacterial exacerbations of Chronic Obstructive Pulmonary Disease (COPD) and Pneumonia.
Uncomplicated Skin and Skin Structure Infections: Due to Staphylococcus aureus, Streptococcus pyogenes, or Streptococcus agalactiae. Abscesses usually require surgical drainage.
Genitourinary Infections: Infections due to Chlamydia trachomatis like Nongonococcal
Urethritis and Cervicitis.
Side Effect
Azithromycin (ZEEKAN) is well tolerated with a low incidence of side effects. Majority of the side effects were mild to moderate in nature and of gastrointestinal in origin with nausea, abdominal discomfort, vomiting, flatulence and diarrhea. Allergic reactions such as rash have occurred and there have also been rare reports of serious hypersensitivity reactions. Reversible elevations in liver transaminases have been seen with a frequency similar to the comparative Macrolides and Penicillins used in clinical trials. Transient mild reductions in neutrophil counts have occasionally been observed in clinical trials, although a causal relationship to Azithromycin (ZEEKAN) has not been established.

Azic 250 mg

Azic 500 mg
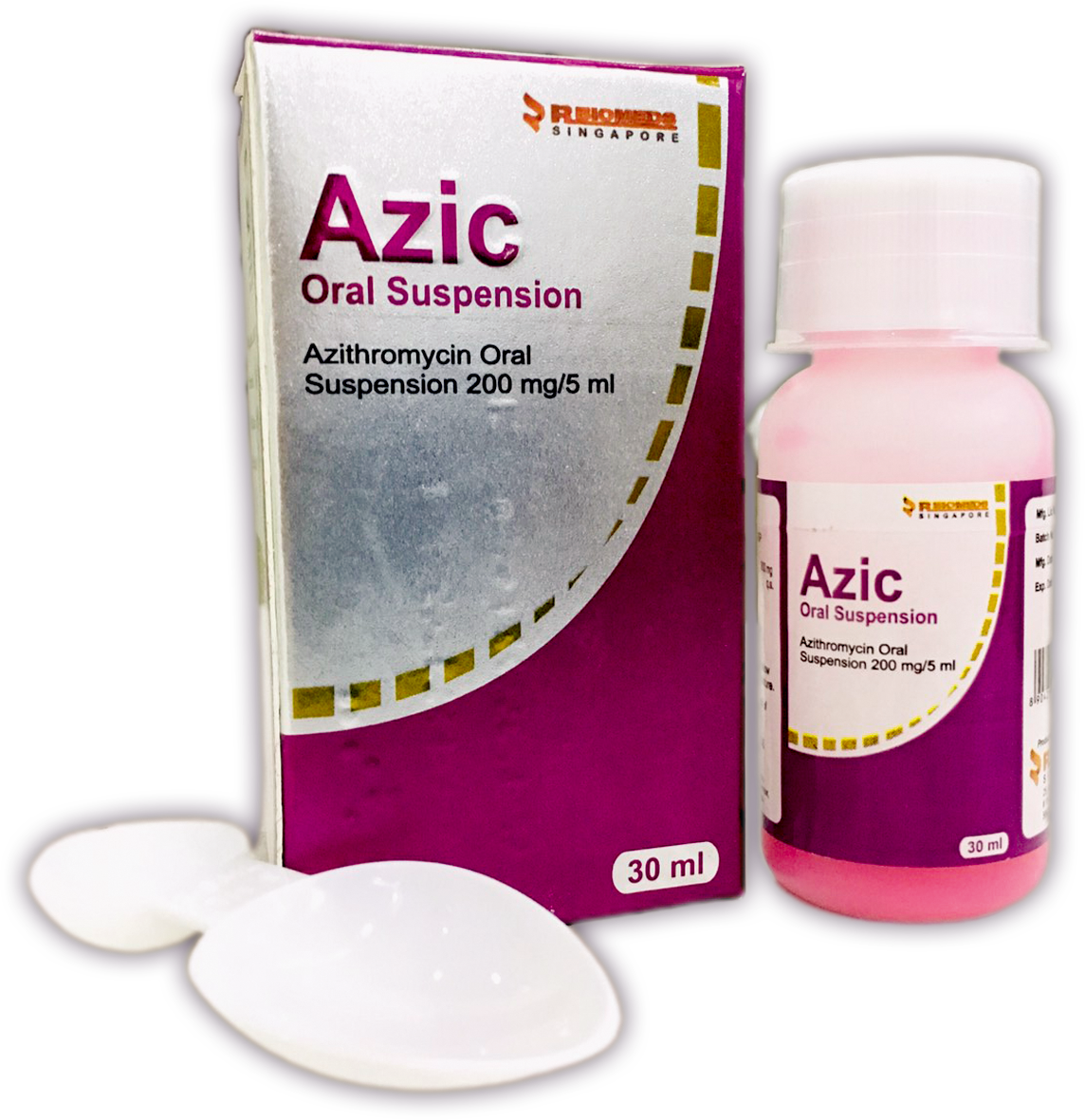
Azic Oral Suspension

Acylex 200mg
Acylex 400mg

Carton Quench Cream 15gm
Icon Itraconazole
Clanoxy 375

Clanoxy 625
Theozee 250 mg

Theoflex 125mg

Clobmarksans cream 10gm
Cloetamark cream 15gm
Izol 100mg

Levcian 500mg Tab

Markvir cream 5gm
Roxiten 150mg

Samaxon Powder for Injection

Samsaf Powder for Injection
Sezo-B Cream

VIRQ 250mg Injection


Zeftahl Powder for Injection


BioVit Tablets

CalciVit Plus Tablets
Calcivit/Calcivit Plus Tab
Composition
Each CalciVit Tablet contains:
Calcium (as Calcium carbonate from natural origin) BP 400mg
Cholecalciferol from natural origin (BP) 4001U.
Each CalciVit Plus Tablet contains:
Calcium carbonate (BP) 400mg, Vitamin D (BP) 400IU Magnesium (as magnesium Sulphate) BP 100mg, Zinc (as Zinc Sulphate) BP 4mg.
Uses
- Promotes health and strength of bones
- Helps to build strong bones and teeth
- Reduces the risk of osteoporosis
- Supports healthy muscle contraction
- Prevents fractures of the hip, vertebrae, wrist, pelvis, ribs, and other bones.
Side Effect
Allergic reactions (like skin rash, itching or hives, swelling of the face, lips, or tongue), Constipation, Flatulence, Nausea, Abdominal pain, Diarrhea, Confusion, Dry mouth, High blood pressure. Increased thirst, Increased urination, Irregular heartbeat, Metallic taste, Muscle or bone pain, Pain when urinating, Seizure, Unusually weak or tired and Weight loss.

CalciVit Tablets
Calcivit/Calcivit Plus Tab
Composition
Each CalciVit Tablet contains:
Calcium (as Calcium carbonate from natural origin) BP 400mg
Cholecalciferol from natural origin (BP) 4001U.
Each CalciVit Plus Tablet contains:
Calcium carbonate (BP) 400mg, Vitamin D (BP) 400IU Magnesium (as magnesium Sulphate) BP 100mg, Zinc (as Zinc Sulphate) BP 4mg.
Uses
- Promotes health and strength of bones
- Helps to build strong bones and teeth
- Reduces the risk of osteoporosis
- Supports healthy muscle contraction
- Prevents fractures of the hip, vertebrae, wrist, pelvis, ribs, and other bones.
Side Effect
Allergic reactions (like skin rash, itching or hives, swelling of the face, lips, or tongue), Constipation, Flatulence, Nausea, Abdominal pain, Diarrhea, Confusion, Dry mouth, High blood pressure. Increased thirst, Increased urination, Irregular heartbeat, Metallic taste, Muscle or bone pain, Pain when urinating, Seizure, Unusually weak or tired and Weight loss.

DiaVit Tablets
DiaVit
Composition
Each DiabeVit Tablet contains:
Vitamin A (BP) 700mcg, Cholecalciferol(BP) 5mcg,Thiamine HCL BP)15mg, Riboflavin (BP) 5mg, Pyridoxine HCI (BP) 25mg, Cyanocobalamin (BP) 9mcg, Nicotinamide (BP) 45mg, Calcium D Pantothenate (BP)10mg, Vitamin C (BP) 120mg, Vitamin E (BP) 30mg, Iron (as Ferrous Fumarate (BP) 8mg, Chromium (as chromium chloride (USP) 0.2mg, Selenium [as sodium selenite (USP)] 0.1mg, Iodine [as potassium iodide (BP)] 0.1mg, Manganese [as Manganese Sulphate (BP)] 2mg, Copper[ as copper Sulphate (BP)] 1mg, Magnesium [as Magnesium Sulphate (B.P)] 100mg, Zinc [as Zinc Sulphate (BP)] 15mg, Folic acid (BP) 0.5mg, Biotin (BP) 0.2mg.
Uses
- Lowers blood sugar and insulin levels
- Improves blood-glucose and lipid levels
- Protects against beta cell destruction
- Helps to protect nerve function
- Improves antioxidant and immune function
- Prevents complication associated with diabetes
- Provides essential vitamins and minerals for daily nutritional support
Side Effect
No information on side effects has been reported.

MuVit G Tablets
MuVit G
Each MuVit G Tablet Contains:
Vitamin A (As Acetate) 0.787mg, Vitamin C (Ascorbic Acid) 52.5mg, Vitamin D (Cholecalciferol) 0.0011mg, Vitamin E (Alphatocopherol) 1.34mg, Vitamin B1 (Thiamine HCI) 1.575mg, Vitamin B2 (Riboflavin) 1.575mg, Nicotinamide 21mg, Vitamin B6 (Pyridoxine HCI) 2.554mg, Vitamin B12 (Cyanocobalamin) 0.0052mg, Calcium D Pantothenate 10.5mg Calcium (Dicalcium Phosphate) 52.5mg Iron (Ferrous Fumarate) 15.75mg, Phosphorous (Dibasic Sodium Phosphate) 22.45mg, Iodine (Potassium Iodide) 0.157mg, Magnesium (Magnesium Sulphate) 6.297mg, Zinc (Zinc Sulphate) 1.75mg, Cobalt (Cobalt Chloride) 0.104 mcg, Copper (Copper Sulphate) 1.641mg, Manganese (Manganese Sulphate) 1.05 mg, Ginseng Exrtract 40mg.
Uses
Muvit G is a comprehensive daily food supplement that has a balanced combination of vitamins, minerals and ginseng. Ginseng is a herbal extract, commonly used to improve well-being, reduce fatigue and enhance the ability to cope with stress. Its balanced combination rejuvenates and strengthens body organs and boosts physical and mental vitality.
Side Effect
The most common side effect is trouble sleeping (insomnia). Less commonly, people experience increased heart rate, high or low blood pressure, headache, loss of appetite, diarrhea, itching, rash, dizziness, and mood changes. Women may also experience swollen breasts, menstrual problems and vaginal bleeding.

A2Z Capsules

A2Z Drops for children

A2Z Syrup for children
Antoxid
Bio E
Cal D3 Tablets

Cal D3 Syrup
Dermavit
Mcobalmin ODS
Vit D3 Drops 200 IU
GLA (soft gel)

Hi Cal Tablet
Hi Cal Syrup

I-Vit soft gel Cap
Tendocian Tab

Vitagen Vitamin C Chewable Tab

Vitagen Zinc Chelate Tab

BioVit Tablets

CalciVit Plus Tablets
Calcivit/Calcivit Plus Tab
Composition
Each CalciVit Tablet contains:
Calcium (as Calcium carbonate from natural origin) BP 400mg
Cholecalciferol from natural origin (BP) 4001U.
Each CalciVit Plus Tablet contains:
Calcium carbonate (BP) 400mg, Vitamin D (BP) 400IU Magnesium (as magnesium Sulphate) BP 100mg, Zinc (as Zinc Sulphate) BP 4mg.
Uses
- Promotes health and strength of bones
- Helps to build strong bones and teeth
- Reduces the risk of osteoporosis
- Supports healthy muscle contraction
- Prevents fractures of the hip, vertebrae, wrist, pelvis, ribs, and other bones.
Side Effect
Allergic reactions (like skin rash, itching or hives, swelling of the face, lips, or tongue), Constipation, Flatulence, Nausea, Abdominal pain, Diarrhea, Confusion, Dry mouth, High blood pressure. Increased thirst, Increased urination, Irregular heartbeat, Metallic taste, Muscle or bone pain, Pain when urinating, Seizure, Unusually weak or tired and Weight loss.

CalciVit Tablets
Calcivit/Calcivit Plus Tab
Composition
Each CalciVit Tablet contains:
Calcium (as Calcium carbonate from natural origin) BP 400mg
Cholecalciferol from natural origin (BP) 4001U.
Each CalciVit Plus Tablet contains:
Calcium carbonate (BP) 400mg, Vitamin D (BP) 400IU Magnesium (as magnesium Sulphate) BP 100mg, Zinc (as Zinc Sulphate) BP 4mg.
Uses
- Promotes health and strength of bones
- Helps to build strong bones and teeth
- Reduces the risk of osteoporosis
- Supports healthy muscle contraction
- Prevents fractures of the hip, vertebrae, wrist, pelvis, ribs, and other bones.
Side Effect
Allergic reactions (like skin rash, itching or hives, swelling of the face, lips, or tongue), Constipation, Flatulence, Nausea, Abdominal pain, Diarrhea, Confusion, Dry mouth, High blood pressure. Increased thirst, Increased urination, Irregular heartbeat, Metallic taste, Muscle or bone pain, Pain when urinating, Seizure, Unusually weak or tired and Weight loss.

DiaVit Tablets
DiaVit
Composition
Each DiabeVit Tablet contains:
Vitamin A (BP) 700mcg, Cholecalciferol(BP) 5mcg,Thiamine HCL BP)15mg, Riboflavin (BP) 5mg, Pyridoxine HCI (BP) 25mg, Cyanocobalamin (BP) 9mcg, Nicotinamide (BP) 45mg, Calcium D Pantothenate (BP)10mg, Vitamin C (BP) 120mg, Vitamin E (BP) 30mg, Iron (as Ferrous Fumarate (BP) 8mg, Chromium (as chromium chloride (USP) 0.2mg, Selenium [as sodium selenite (USP)] 0.1mg, Iodine [as potassium iodide (BP)] 0.1mg, Manganese [as Manganese Sulphate (BP)] 2mg, Copper[ as copper Sulphate (BP)] 1mg, Magnesium [as Magnesium Sulphate (B.P)] 100mg, Zinc [as Zinc Sulphate (BP)] 15mg, Folic acid (BP) 0.5mg, Biotin (BP) 0.2mg.
Uses
- Lowers blood sugar and insulin levels
- Improves blood-glucose and lipid levels
- Protects against beta cell destruction
- Helps to protect nerve function
- Improves antioxidant and immune function
- Prevents complication associated with diabetes
- Provides essential vitamins and minerals for daily nutritional support
Side Effect
No information on side effects has been reported.

MuVit G Tablets
MuVit G
Each MuVit G Tablet Contains:
Vitamin A (As Acetate) 0.787mg, Vitamin C (Ascorbic Acid) 52.5mg, Vitamin D (Cholecalciferol) 0.0011mg, Vitamin E (Alphatocopherol) 1.34mg, Vitamin B1 (Thiamine HCI) 1.575mg, Vitamin B2 (Riboflavin) 1.575mg, Nicotinamide 21mg, Vitamin B6 (Pyridoxine HCI) 2.554mg, Vitamin B12 (Cyanocobalamin) 0.0052mg, Calcium D Pantothenate 10.5mg Calcium (Dicalcium Phosphate) 52.5mg Iron (Ferrous Fumarate) 15.75mg, Phosphorous (Dibasic Sodium Phosphate) 22.45mg, Iodine (Potassium Iodide) 0.157mg, Magnesium (Magnesium Sulphate) 6.297mg, Zinc (Zinc Sulphate) 1.75mg, Cobalt (Cobalt Chloride) 0.104 mcg, Copper (Copper Sulphate) 1.641mg, Manganese (Manganese Sulphate) 1.05 mg, Ginseng Exrtract 40mg.
Uses
Muvit G is a comprehensive daily food supplement that has a balanced combination of vitamins, minerals and ginseng. Ginseng is a herbal extract, commonly used to improve well-being, reduce fatigue and enhance the ability to cope with stress. Its balanced combination rejuvenates and strengthens body organs and boosts physical and mental vitality.
Side Effect
The most common side effect is trouble sleeping (insomnia). Less commonly, people experience increased heart rate, high or low blood pressure, headache, loss of appetite, diarrhea, itching, rash, dizziness, and mood changes. Women may also experience swollen breasts, menstrual problems and vaginal bleeding.

A2Z Capsules

A2Z Drops for children

A2Z Syrup for children

Antoxid

Bio E

Cal D3 Tablets

Cal D3 Syrup

Dermavit

Mcobalmin ODS

Vit D3 Drops 200 IU

GLA (soft gel)

Hi Cal Tablet

Hi Cal Syrup

I-Vit soft gel Cap

Tendocian Tab

Vitagen Vitamin C Chewable Tab

Vitagen Zinc Chelate Tab

Sitafer Tablets
Sitafer 50 mg/100 mg
Composition
Each tablet contains SitagIiptin Phosphate 50 mg / 100 mg
Uses
Monotherapy and combination therapy
Sitafer is Indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Side Effect
Upper respiratory tract Infection, nasopharyngitis and headache
Valiant Tablets
Valiant
Each film coated tablet contains:
Vildagliptin 50mg
Uses
Vildagliptin is indicated in the treatment of type 2 diabetes mellitus in adults:
As monotherapy
In patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to contraindications or intolerance.
As dual oral therapy in combination with Metformin, in patients with insufficient glycemic control despite maximal tolerated dose of monotherapy with metformin.
A sulphonylurea, in patients with insufficient glycemic control despite maximal tolerated dose of a sulphonylurea and for whom metformin is inappropriate due to contraindications or intolerance.
A thiazolidinedione, in patients with insufficient glycemic control and for whom the use of a thiazolidinedione is appropriate.
As triple oral therapy in combination with
A sulphonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycemic control.
Vildagliptin is also indicated for use in combination with insulin (with or without metformin) when diet and exercise plus a stable dose of insulin do not provide adequate glycemic control.
Side Effect
Common: Dizziness
Uncommon: Headache, constipation, swollen hands, ankle or feet edema, joint pain, low blood glucose.
Rare: Angioedema, Hepatitis
Very rare: Sore throat, runny nose, fever

Sitafer Tablets
Sitafer 50 mg/100 mg
Composition
Each tablet contains SitagIiptin Phosphate 50 mg / 100 mg
Uses
Monotherapy and combination therapy
Sitafer is Indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Side Effect
Upper respiratory tract Infection, nasopharyngitis and headache

Valiant Tablets
Valiant
Each film coated tablet contains:
Vildagliptin 50mg
Uses
Vildagliptin is indicated in the treatment of type 2 diabetes mellitus in adults:
As monotherapy
In patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to contraindications or intolerance.
As dual oral therapy in combination with Metformin, in patients with insufficient glycemic control despite maximal tolerated dose of monotherapy with metformin.
A sulphonylurea, in patients with insufficient glycemic control despite maximal tolerated dose of a sulphonylurea and for whom metformin is inappropriate due to contraindications or intolerance.
A thiazolidinedione, in patients with insufficient glycemic control and for whom the use of a thiazolidinedione is appropriate.
As triple oral therapy in combination with
A sulphonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycemic control.
Vildagliptin is also indicated for use in combination with insulin (with or without metformin) when diet and exercise plus a stable dose of insulin do not provide adequate glycemic control.
Side Effect
Common: Dizziness
Uncommon: Headache, constipation, swollen hands, ankle or feet edema, joint pain, low blood glucose.
Rare: Angioedema, Hepatitis
Very rare: Sore throat, runny nose, fever

Flosure Capsules
Flosure
Each Flosure capsule contains :
Tamsulosin Hydrochloride USP 0.4 mg
Uses
Flosure (tamsulosin HCI) capsules are indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH).
Side Effect
Central nervous system: Headache, dizziness insomnolence, insomnia ,vertigo, Genitourinary: Abnormal ejaculation, Respiratory: Rhinitis, pharyngitis, cough, sinusitis, Cardiovascular: Chest pain, orthostatic hypotension, Endocrine & metabolic: Libido decreased ,Gastrointestinal: Diarrhea, nausea, Neuromuscular & skeletal: Weakness, back pain, Ocular: Blurred vision, Miscellaneous: Infections, <1% (Limited to important or life-threatening): Allergic reactions: angioedema, pruritus, rash, urticaria, respiratory symptoms; constipation, palpitation, priapism, syncope, hypotension, orthostasis (symptomatic) skin desquamation, vomiting.
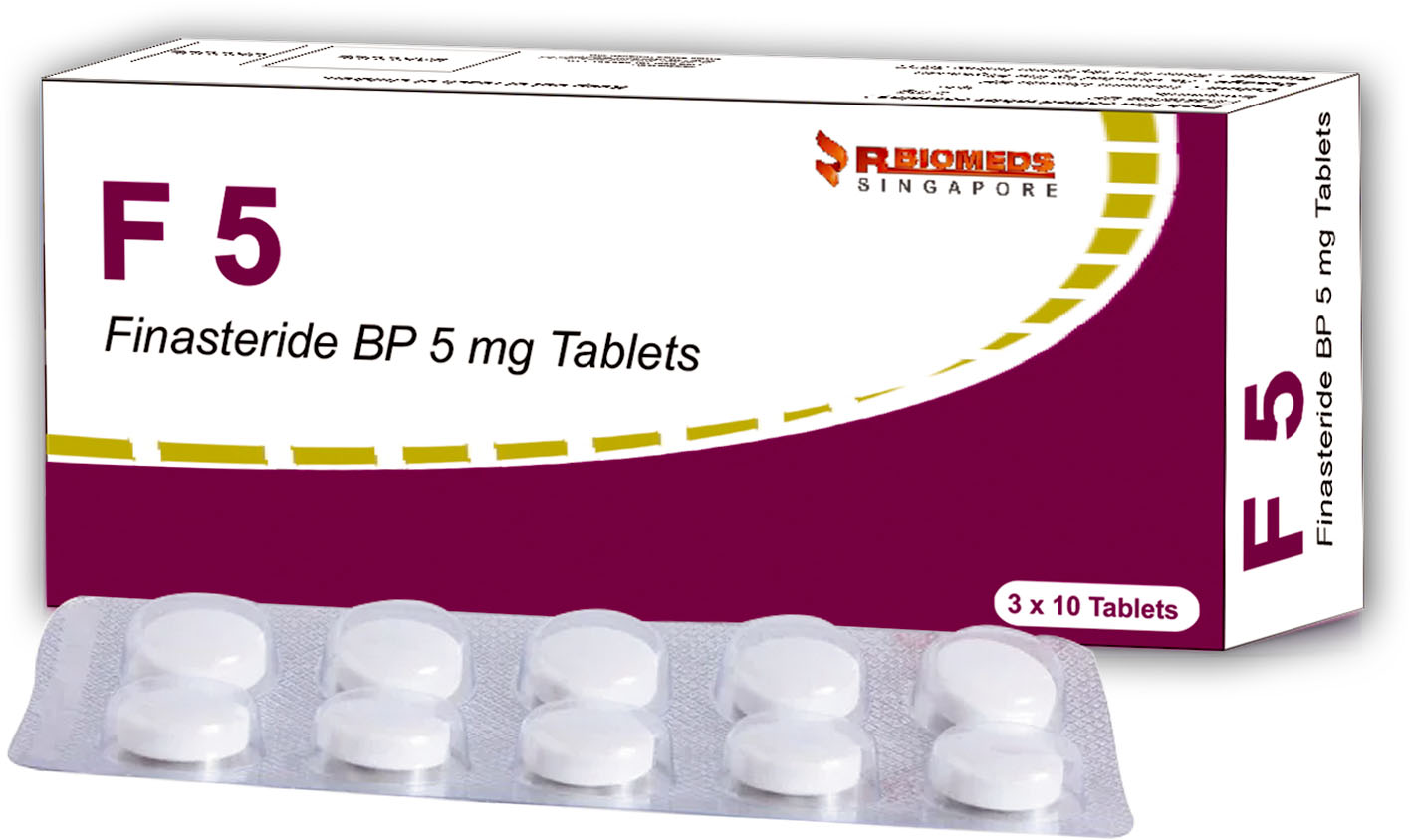
F5 Tab

Flosure Capsules
Flosure
Each Flosure capsule contains :
Tamsulosin Hydrochloride USP 0.4 mg
Uses
Flosure (tamsulosin HCI) capsules are indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH).
Side Effect
Central nervous system: Headache, dizziness insomnolence, insomnia ,vertigo, Genitourinary: Abnormal ejaculation, Respiratory: Rhinitis, pharyngitis, cough, sinusitis, Cardiovascular: Chest pain, orthostatic hypotension, Endocrine & metabolic: Libido decreased ,Gastrointestinal: Diarrhea, nausea, Neuromuscular & skeletal: Weakness, back pain, Ocular: Blurred vision, Miscellaneous: Infections, <1% (Limited to important or life-threatening): Allergic reactions: angioedema, pruritus, rash, urticaria, respiratory symptoms; constipation, palpitation, priapism, syncope, hypotension, orthostasis (symptomatic) skin desquamation, vomiting.

F5 Tab


Lidomark Gel
Lidomark
Composition
Each gram contains Lidocaine Hydrochloride BP (anhydrous) 2.0% w/v, Methyl Paraben BP (as preservative) 0.10% w/v, Propyl Paraben BP (as preservative) 0.05% w/v
Uses
LIDOMARK is indicated for the prevention and relief of pain caused by:
minor skin irritations
minor burns, including sunburn
anorectal pain
minor cuts
insect bites
Side Effect
Drowsiness, blurred vision, tremor, altered tastes, Tinnitus
Bupiright S

Lidomark Gel
Lidomark
Composition
Each gram contains Lidocaine Hydrochloride BP (anhydrous) 2.0% w/v, Methyl Paraben BP (as preservative) 0.10% w/v, Propyl Paraben BP (as preservative) 0.05% w/v
Uses
LIDOMARK is indicated for the prevention and relief of pain caused by:
minor skin irritations
minor burns, including sunburn
anorectal pain
minor cuts
insect bites
Side Effect
Drowsiness, blurred vision, tremor, altered tastes, Tinnitus

Bupiright S


Tocilizumab
Fligen 300 mcg

Rilast 100 mg

Rilast 500 mg

Asparag-Med

Doxomark 10mg

Doxomark 50mg
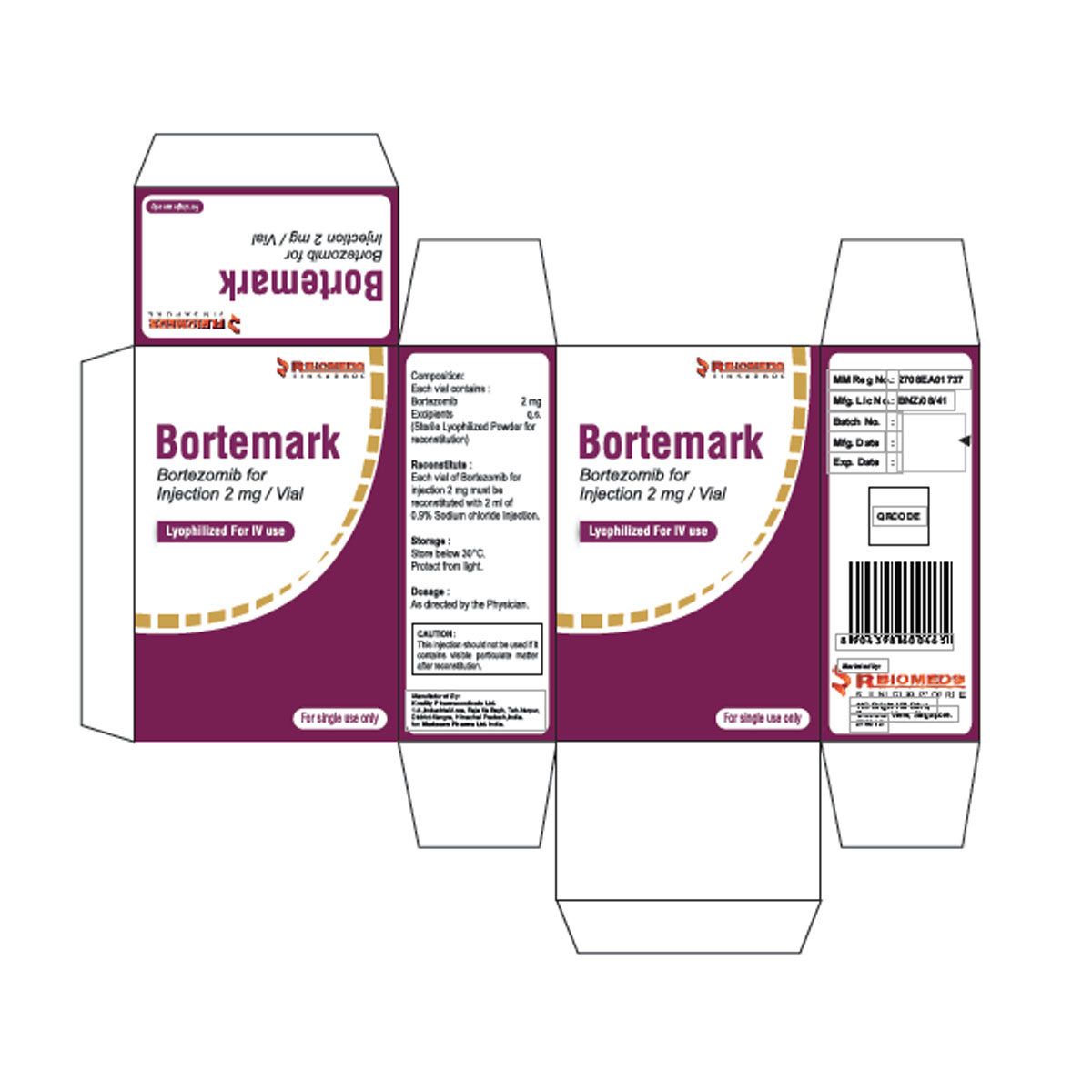
Bortemark 2mg

Bortemark 3.5mg

Lenalid 5
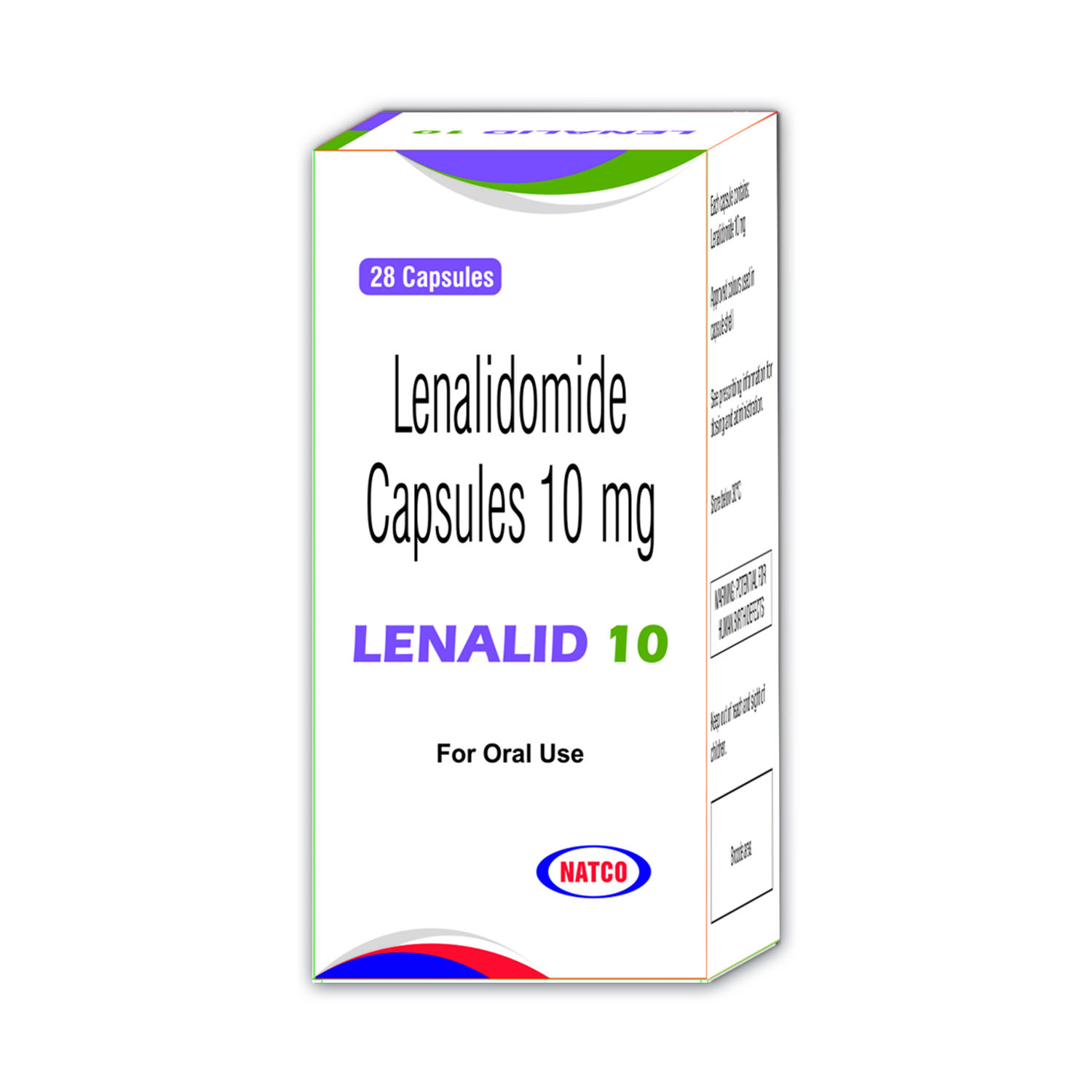
Lenalid 5

Maturus Azacitidine 100 mg
Xpreza 100 Ing


Tocilizumab
Theofenac 100 mg
Theofenac Tab
Composition
Each film coated tablet contains:
Aceclofenac BP 100 mg
Excipients q.s.
Approved colours used
Each film coated sustained
release tablet contains:
Aceclofenac BP 200 mg
Excipients
Approved colours used
Uses
Aceclofenac is indicated for the relief of pain and inflammation in osteoarthritis, rheumatoid arthritis and ankylosing spondylitis.
Side Effect
Gastrointestinal:
The most commonly-observed adverse events are gastrointestinal in nature. Peptic ulcers, perforation or GI bleeding, sometimes fatal, particularly in the elderly, may occur. Nausea, vomiting, diarrhea, flatulence, constipation, dyspepsia, abdominal pain, melaena, hematemesis, ulcerative stomatitis, exacerbation of colitis and Crohn’s disease have been reported following administration. Less frequently, gastritis has been observed. Pancreatitis has bran reposed very rarely.
Hypersensitivity:
Hypersensitivity reaction have been reported following treatment with NSAIDs. These may consist of:
(a) Non-specific allergic reactions and anaphylaxis
(b) Respiratory tract reactivity comprising asthma, aggravated asthma, bronchospasm or dyspnea,
(c) Assorted skin disorders, including rashes of various types, pruritus, urticaria, purpose, angioedema and, more rarely exfoliative and bullous dermatoses (including epidermal necrolysis and erythema multiforme).
Cardiovascular:
Oedema, hypertension and cardiac failure have been reported in association with NSAID treatment.
Other adverse reactions reported less commonly include:
Renal:
Nephrotoxicity in various forms, including interstitial nephritis, nephritic syndrome and renal failure.
Hepatic:
Abnormal liver function, hepatitis and jaundice.
Theofenac 200 mg
Theofenac Tab
Composition
Each film coated tablet contains:
Aceclofenac BP 100 mg
Excipients q.s.
Approved colours used
Each film coated sustained
release tablet contains:
Aceclofenac BP 200 mg
Excipients
Approved colours used
Uses
Aceclofenac is indicated for the relief of pain and inflammation in osteoarthritis, rheumatoid arthritis and ankylosing spondylitis.
Side Effect
Gastrointestinal:
The most commonly-observed adverse events are gastrointestinal in nature. Peptic ulcers, perforation or GI bleeding, sometimes fatal, particularly in the elderly, may occur. Nausea, vomiting, diarrhea, flatulence, constipation, dyspepsia, abdominal pain, melaena, hematemesis, ulcerative stomatitis, exacerbation of colitis and Crohn’s disease have been reported following administration. Less frequently, gastritis has been observed. Pancreatitis has bran reposed very rarely.
Hypersensitivity:
Hypersensitivity reaction have been reported following treatment with NSAIDs. These may consist of:
(a) Non-specific allergic reactions and anaphylaxis
(b) Respiratory tract reactivity comprising asthma, aggravated asthma, bronchospasm or dyspnea,
(c) Assorted skin disorders, including rashes of various types, pruritus, urticaria, purpose, angioedema and, more rarely exfoliative and bullous dermatoses (including epidermal necrolysis and erythema multiforme).
Cardiovascular:
Oedema, hypertension and cardiac failure have been reported in association with NSAID treatment.
Other adverse reactions reported less commonly include:
Renal:
Nephrotoxicity in various forms, including interstitial nephritis, nephritic syndrome and renal failure.
Hepatic:
Abnormal liver function, hepatitis and jaundice.

Celekop 100 mg

Celekop 200 mg


Tocilizumab

Esomega 20 mg Cap
Esomega
Each capsule contains:
1.Enteric coated pellets of Esomeprazole magnesium
trihydrate U.S.P. eq. to Esomeprazole………20mg
2.Enteric coated pellets of Esomeprazole magnesium
trihydrate U.S.P. eq. to Esomeprazole……… 40mg
Uses
ESOMEGA is indicated for the treatment of Treatment of Gastroesophageal Reflux Disease (GERD), Symptomatic Gastroesophageal Reflux Disease, NSAID-Associated Gastric Ulcer, H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence Triple Therapy, Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome, Prolonged treatment after I.V. induced prevention of rebleeding of peptic ulcers.
Side Effect
Central nervous system: Headache (2% to 11%), Irritability (infants: 55%), dizziness (<1%), vertigo, drowsiness (children: 2%; adults: <1%)
Dermatologic: Pruritus (<1%)
Endocrine & metabolic: Altered thyroid hormone levels (increased thyroxine: 01%), decreased serum potassium (51%), decreased serum sodium (01./0), decreased thyroid hormones (thyroxine: 51%), increased gastrin (01%), increased serum potassium (01%), increased serum sodium (01%), increased thyroid stimulating hormone level (01./0), increased uric acid (010/0)
Gastrointestinal: Flatulence (51%), diarrhea (2% to 4%), abdominal pain (1% to 6%), nausea (oral: 51% to 2%), vomiting (infants: 1% to ,5%; adults: <1%), xerostomia (,1°/0), constipation (oral: ,1%)
Hematologic &oncologic: Change in platelet count (,1%)
Hepatic: Increased serum alkaline phosphatase (010/0), increased serum ALT (010/0), increased serum AST (010/0) Renal: Increased serum creatinine (51./o)
Respiratory: Cough (<1%), tachypnea (infants: 1%)
Miscellaneous: Fever (<1./0)
Cardiovascular: Esophageal varices
Gastrointestinal: Barrett esophagus, duodenitis, esophageal stenosis, esophageal ulcer, esophagitis, gastritis, mucosal discoloration
Hematologic & oncologic: Benign polyp
Miscellaneous: Benign nodule.

Esomega 40gm Cap
Esomega
Each capsule contains:
1.Enteric coated pellets of Esomeprazole magnesium
trihydrate U.S.P. eq. to Esomeprazole………20mg
2.Enteric coated pellets of Esomeprazole magnesium
trihydrate U.S.P. eq. to Esomeprazole……… 40mg
Uses
ESOMEGA is indicated for the treatment of Treatment of Gastroesophageal Reflux Disease (GERD), Symptomatic Gastroesophageal Reflux Disease, NSAID-Associated Gastric Ulcer, H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence Triple Therapy, Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome, Prolonged treatment after I.V. induced prevention of rebleeding of peptic ulcers.
Side Effect
Central nervous system: Headache (2% to 11%), Irritability (infants: 55%), dizziness (<1%), vertigo, drowsiness (children: 2%; adults: <1%)
Dermatologic: Pruritus (<1%)
Endocrine & metabolic: Altered thyroid hormone levels (increased thyroxine: 01%), decreased serum potassium (51%), decreased serum sodium (01./0), decreased thyroid hormones (thyroxine: 51%), increased gastrin (01%), increased serum potassium (01%), increased serum sodium (01%), increased thyroid stimulating hormone level (01./0), increased uric acid (010/0)
Gastrointestinal: Flatulence (51%), diarrhea (2% to 4%), abdominal pain (1% to 6%), nausea (oral: 51% to 2%), vomiting (infants: 1% to ,5%; adults: <1%), xerostomia (,1°/0), constipation (oral: ,1%)
Hematologic &oncologic: Change in platelet count (,1%)
Hepatic: Increased serum alkaline phosphatase (010/0), increased serum ALT (010/0), increased serum AST (010/0) Renal: Increased serum creatinine (51./o)
Respiratory: Cough (<1%), tachypnea (infants: 1%)
Miscellaneous: Fever (<1./0)
Cardiovascular: Esophageal varices
Gastrointestinal: Barrett esophagus, duodenitis, esophageal stenosis, esophageal ulcer, esophagitis, gastritis, mucosal discoloration
Hematologic & oncologic: Benign polyp
Miscellaneous: Benign nodule.
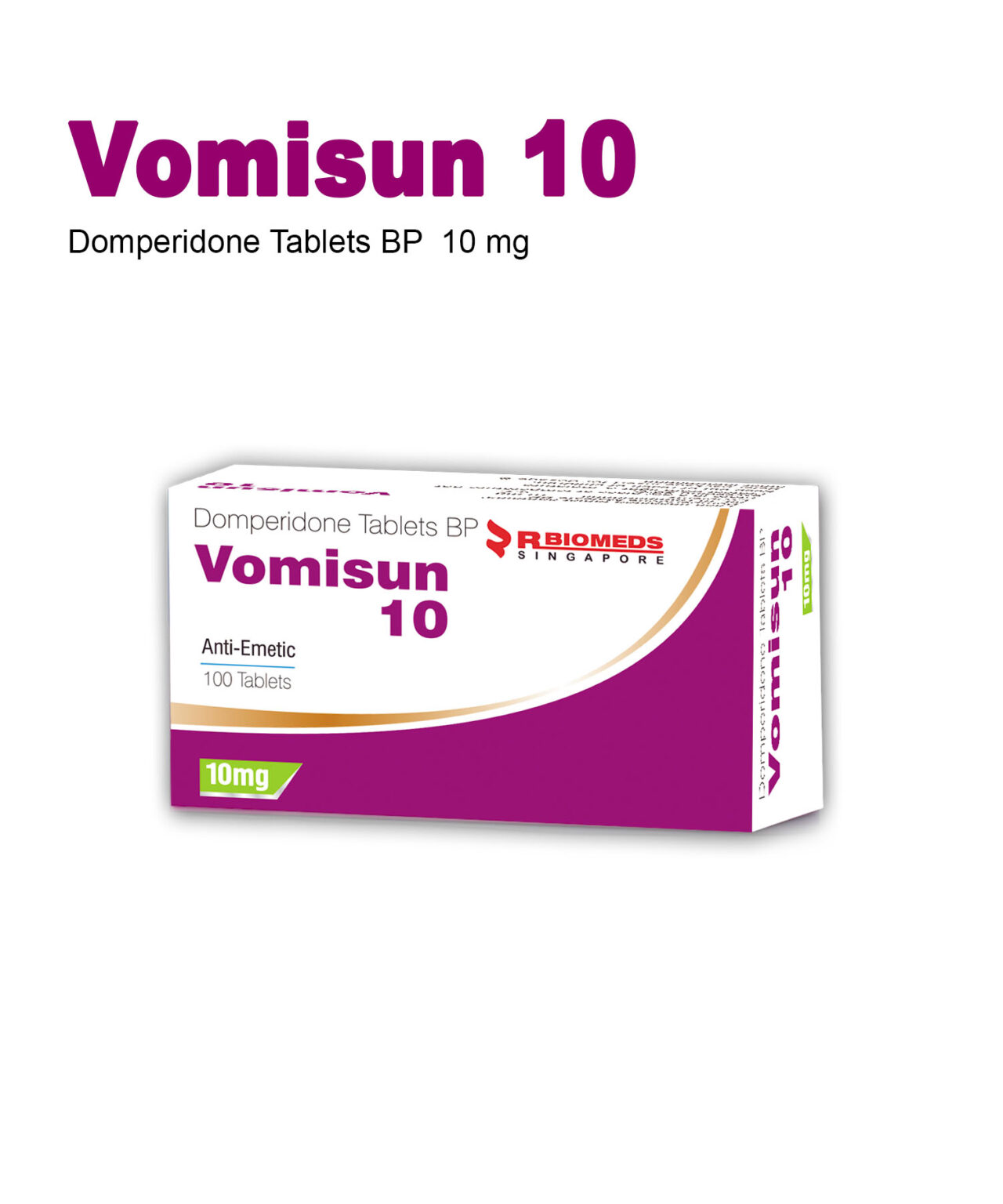
Vomisun 10mg Tab
Vomisun
Composition
Each uncoated Tablet contains domperidone maleate BP
e.q. to domperidone 10mg
Excipients q.s
Uses
Vomisun is indicated for the relief of the symptoms of nausea and vomiting.
Side Effect
Allergic reactions such as skin rash, itching, shortness of breath, wheezing and/or swollen face, irregular heartbeat abnormal muscle movements or posture. tremor (shaking), muscle stiffness or unusual eye movements.
Leaking of milky fluid from the breasts sore or painful breasts decreased sex drive weakness mild skin rash or hives
Domperidone may be associated with an increased risk of heart rhythm disorder and cardiac arrest.
This risk may be more m most over 60 years old or taking doses higher than 30 mg per day.

Dynetic 50 mg Tab
Dynetic
Each film coated tablet contains: Itopride HC1 50mg; Inactive Ingredients: Colloidal Anhydrous Silica; Croscarmellose Sodium; Magnesium Stearate; Talcum; Lactose; Microcrystalline Cellulose; Opadry Orange 85G53561; Carnauba Wax
Uses
Dynetic tablets are indicated for the treatment of
- Functional dyspepsia
- Non-Ulcer Dyspepsia (chronic gastritis) i.e. sensation of bloating, early satiety, upper abdominal pain or discomfort, anorexia, heartburn, nausea and vomiting.
Side Effect
The following adverse events have been reported in patients receiving Itopride hydrochloride.
Blood and lymphatic system disorders:- leukopenia and thrombocytopenia.
Immune system disorders Anaphylactoid reaction.
Endocrine disorders:- increased prolactin level and gynecomastia.
Nervous system disorders :- Dizziness, headache and tremor.
Gastrointestinal disorders:- Diarrhea, constipation, abdominal pain, increased saliva and nausea.
Hepato-biliary disorders:- jaundice.
Skin and subcutaneous tissue disorders:- Rash, redness and itching.
Investigations Increased AST (SGOT), increased ALT (SGPT), increased gamma-(GTP), increased alkaline phosphatase, and increased bilirubin.

Ondan MDS 4 mg
Ondan MDS
Each orally disintegrating strip contains:
Ondansetron Hydrochloride USP
Eq. to Ondansetron 4/8 mg
Excipients Q.S.
Uses
Ondansetron is indicated for the management of nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy.
Ondansetron is indicated for the prevention of post-operative nausea and vomiting (PONV).
Side Effect
Headache, Sensation of warmth or flushing, Constipation, Seizures, movement disorders (including extrapyramidal reactions such as dystonic reactions, oculogyric crisis and dyskinesia), Arrhythmias, chest pain with or without ST segment depression, bradycardia, Hypotension, Hiccups, Asymptomatic increases in liver function tests.

Ondan MDS 8 mg
Ondan MDS
Each orally disintegrating strip contains:
Ondansetron Hydrochloride USP
Eq. to Ondansetron 4/8 mg
Excipients Q.S.
Uses
Ondansetron is indicated for the management of nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy.
Ondansetron is indicated for the prevention of post-operative nausea and vomiting (PONV).
Side Effect
Headache, Sensation of warmth or flushing, Constipation, Seizures, movement disorders (including extrapyramidal reactions such as dystonic reactions, oculogyric crisis and dyskinesia), Arrhythmias, chest pain with or without ST segment depression, bradycardia, Hypotension, Hiccups, Asymptomatic increases in liver function tests.

Ifaxa 200 mg Tab
Ifaxa
Each film coated tablet contains: Rifaximin 200mg and 550mg respectively; inactive ingredients: Povidone; Crosscarmellose Sodium; Colloidal Anhydrous Silica; Magnesium Stearate; Talcum; Lactose; Microcrystalline Cellulose; Opadry Orange 200F230017 (Ifaxa 200mg); Opadry Brown 200F265003 (Ifaxa 550mg).
Uses
IFAXA 200 mg is indicated for the treatment of patients (>12 years of age) with travelers’ diarrhea caused by noninvasive strains of Escherichia coli.
IFAXA 550 mg is indicated for reduction in risk of overt hepatic encephalopathy (HE) recurrence in patients 18 years of age.
Side Effect
Cardiovascular: Peripheral edema (15%), Central nervous system: Dizziness (13%), fatigue (12%)
Hepatic: Ascites (11%). Gastrointestinal: Nausea (14%)
2% to 10%:
Cardiovascular: Chest pain (>2% to 5%), hypotension (>2% to 5%. Central nervous system: Headache (10%), depression (7%), fever (6%), amnesia (>2% to 5%), attention disturbance (>2% to 5%), confusion (>2% to 5%), hypoesthesia (>2% to 5%), pain (>2% to 5%), tremor. Dermatological: Pruritus (9%), rash (5%), cellulitis (>2% to 5%). Endocrine and metabolism: Hyper-/hypoglycemia (>2% to 5%), hyperkalemia (>2% to 5%), hyponatremia (>2% to 5%). Gastrointestinal: Abdominal pain (6% to 9%), anorexia (>2% to 5%), dehydration (>2% to 5%), esophageal varices (>2% to 5%), weight gain (>2% to 5%), xerostomia (>2% to 5%). Hematologic: Anemia (8%). Neuromuscular & skeletal: Muscle spasms (9%), arthralgia (6%), myalgia (>2% to 5%). Respiratory: Nasopharyngitis (7%), dyspnea (6%), epistaxis(>2% to
5%), pneumonia (>2% to 5%), rhinitis (>2% to 5%), upper respiratory tract infection (>2% to 5%). Miscellaneous: Influenza-like illness (>2% to 5%)
<2% (Limited to important or life-threatening): Abnormal dreams, allergic dermatitis, anaphylaxis, angioneurotic edema (including tongue and facial edema with dysphagia), CDAD, dysuria, exfoliative dermatitis, flushing, hematuria, hypersensitivity reactions, insomnia, lymphocytosis, monocytosis, motion sickness, neutropenia, polyuria, proteinuria, sunburn, tinnitus, urticaria.

Esomeprazole for injection 40mg

Ezopep 20 mg Tab

Ezopep 40 mg Tab

Ezylax Bottle

Ezylax 50 sachets box
Theopraz plus
Fortigermina

Esoright powder for injection

Novapressin Inj


Tocilizumab

Centaurus 0.5 mg Tab
Centaurus
Composition
Each flim tablet contains Entecavir 0.5mg
Uses
CENTAURUS (entecavir) is indicated for the treatment of chronic hepatitis B virus infection in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease
Side Effect
Most common adverse reactions (<3%, all severity grades) are headache, fatigue, dizziness, and nausea.
Tenofovir Tab


Drools Chicken And Egg Adult 400 gm

Drools Chicken And Egg Puppy 400 gm

Drools Chicken And Egg Puppy 3 Kg

Drools Chicken And Egg Adult 3 Kg

Drools Ocean Fish For Kitten 60 gm

Drools Ocean Fish For Kitten 400 gm

Drools Ocean Fish For Cat 60 gm

Drools Ocean Fist For Cat 400 gm

Drools Tuna Salmon For Cat 400 gm

Drools Real Chicken Biscuits 800 Gms

Drools Absolute Salmon Oil For Pet 150 ml

Drools Chicken And Egg Puppy 21 Kg

Drools Chicken And Egg Adult 21 Kg

Drools Ocean Fish For Kitten 4Kg

Drools Absolute Calcium Bone For Pets 300gm

Drools Chicken And Egg Adult 400 gm

Drools Chicken And Egg Puppy 400 gm

Drools Chicken And Egg Puppy 3 Kg

Drools Chicken And Egg Adult 3 Kg

Drools Ocean Fish For Kitten 60 gm

Drools Ocean Fish For Kitten 400 gm

Drools Ocean Fish For Cat 60 gm

Drools Ocean Fist For Cat 400 gm

Drools Tuna Salmon For Cat 400 gm

Drools Real Chicken Biscuits 800 Gms

Drools Absolute Salmon Oil For Pet 150 ml

Drools Chicken And Egg Puppy 21 Kg

Drools Chicken And Egg Adult 21 Kg

Drools Ocean Fish For Kitten 4Kg

Drools Absolute Calcium Bone For Pets 300gm


Drools Chicken And Egg Adult 400 gm

Drools Chicken And Egg Puppy 400 gm

Drools Chicken And Egg Puppy 3 Kg

Drools Chicken And Egg Adult 3 Kg

Drools Ocean Fish For Kitten 60 gm

Drools Ocean Fish For Kitten 400 gm

Drools Ocean Fish For Cat 60 gm

Drools Ocean Fist For Cat 400 gm

Drools Tuna Salmon For Cat 400 gm

Drools Real Chicken Biscuits 800 Gms

Drools Absolute Salmon Oil For Pet 150 ml

Drools Chicken And Egg Puppy 21 Kg

Drools Chicken And Egg Adult 21 Kg

Drools Ocean Fish For Kitten 4Kg

Drools Absolute Calcium Bone For Pets 300gm

Bioskin hand sanitizer gel


Drools Chicken And Egg Adult 400 gm

Drools Chicken And Egg Puppy 400 gm

Drools Chicken And Egg Puppy 3 Kg

Drools Chicken And Egg Adult 3 Kg

Drools Ocean Fish For Kitten 60 gm

Drools Ocean Fish For Kitten 400 gm

Drools Ocean Fish For Cat 60 gm

Drools Ocean Fist For Cat 400 gm

Drools Tuna Salmon For Cat 400 gm

Drools Real Chicken Biscuits 800 Gms

Drools Absolute Salmon Oil For Pet 150 ml

Drools Chicken And Egg Puppy 21 Kg

Drools Chicken And Egg Adult 21 Kg

Drools Ocean Fish For Kitten 4Kg

Drools Absolute Calcium Bone For Pets 300gm